Clinical and In Vitro Safety of Heyndrickxia coagulans AO 1167B: A Double-Blind, Placebo-Controlled Trial
- PMID: 39770785
- PMCID: PMC11677179
- DOI: 10.3390/microorganisms12122584
Clinical and In Vitro Safety of Heyndrickxia coagulans AO 1167B: A Double-Blind, Placebo-Controlled Trial
Abstract
(1) Background: Heyndrickxia coagulans, a lactic acid-producing bacterium, displays characteristics of both Lactobacillus and Bacillus genera. Clinical evidence suggests its potential health benefits. This study evaluated the safety of H. coagulans AO1167B as a candidate probiotic supplement. (2) Methods: Strain identification was confirmed through morphological, cultural, and genomic analyses, including 16S RNA and whole genome sequencing to assess antimicrobial resistance and virulence factors. Phenotypic tests, such as disk diffusion for antimicrobial resistance, and safety assays for cytotoxicity and hemolytic activity, were conducted. In a phase I, double-blind, placebo-controlled clinical trial, healthy adults were randomized into H. coagulans AO1167B and placebo groups for 60 days. Daily capsule consumption was monitored through clinical and hematological evaluations, adverse event tracking, and health surveys. (3) Results: The genome of H. coagulans AO1167B revealed no concerning features. Disk diffusion tests showed no antimicrobial resistance. The strain exhibited no cytotoxic or hemolytic activity, indicating in vitro safety. No significant differences in clinical or hematological parameters were observed between groups. The most common adverse event, gas, diminished over time. (4) Conclusions: H. coagulans AO1167B demonstrates a suitable safety profile, genetic stability, and probiotic potential for gastrointestinal health, justifying further clinical research.
Keywords: Heyndrickxia coagulans; clinical trial; humans; probiotic; randomized; safety.
Conflict of interest statement
The authors declare no conflicts of interest.
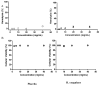
Figures

Similar articles
-
Heyndrickxia coagulans LMG S-24828 Is a Safe Probiotic Strain Capable of Germinating in the Human Gut.Probiotics Antimicrob Proteins. 2024 Oct 21. doi: 10.1007/s12602-024-10383-4. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 39432229
-
In-silico analysis of probiotic attributes and safety assessment of probiotic strain Bacillus coagulans BCP92 for human application.Lett Appl Microbiol. 2024 Jan 2;77(1):ovad145. doi: 10.1093/lambio/ovad145. Lett Appl Microbiol. 2024. PMID: 38148133
-
Progress of research and application of Heyndrickxia coagulans (Bacillus coagulans) as probiotic bacteria.Front Cell Infect Microbiol. 2024 May 28;14:1415790. doi: 10.3389/fcimb.2024.1415790. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38863834 Free PMC article. Review.
-
The effects of Bacillus coagulans MTCC 5856 on functional gas and bloating in adults: A randomized, double-blind, placebo-controlled study.Medicine (Baltimore). 2023 Mar 3;102(9):e33109. doi: 10.1097/MD.0000000000033109. Medicine (Baltimore). 2023. PMID: 36862903 Free PMC article. Clinical Trial.
-
Potential Use of Bacillus coagulans in the Food Industry.Foods. 2018 Jun 13;7(6):92. doi: 10.3390/foods7060092. Foods. 2018. PMID: 29899254 Free PMC article. Review.
Cited by
-
Protective antibodies against enterotoxigenic Escherichia coli are generated from heat-labile toxoid vaccination and exhibit subject- and vaccine-specific diversity.Med Microbiol Immunol. 2025 Feb 11;214(1):10. doi: 10.1007/s00430-025-00817-3. Med Microbiol Immunol. 2025. PMID: 39934422 Free PMC article. Clinical Trial.
References
-
- Hammer B.W. Bacteriological studies on the coagulation of evaporated milk. Iowa Agric. Exp. Stn. Res. Bull. 1915;19:119–131.
-
- Gupta R.S., Patel S., Saini N., Chen S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020;70:5753–5798. - PubMed
-
- Andreoletti O., Budka H., Buncic S., Colin P., Collins J.D., De Koeijer A., Griffin J., Havelaar A., Hope J., Klein G., et al. The maintenance of the list of QPS microorganisms intentionally added to food or feed—Scientific Opinion of the Panel on Biological Hazards. EFSA J. 2008;6:923. doi: 10.2903/j.efsa.2008.923. - DOI
LinkOut - more resources
Full Text Sources

