Innovative Methodology for Antimicrobial Susceptibility Determination in Mycoplasma Biofilms
- PMID: 39770853
- PMCID: PMC11728330
- DOI: 10.3390/microorganisms12122650
Innovative Methodology for Antimicrobial Susceptibility Determination in Mycoplasma Biofilms
Erratum in
-
Correction: Jacobson et al. Innovative Methodology for Antimicrobial Susceptibility Determination in Mycoplasma Biofilms. Microorganisms 2024, 12, 2650.Microorganisms. 2025 Oct 10;13(10):2331. doi: 10.3390/microorganisms13102331. Microorganisms. 2025. PMID: 41156874 Free PMC article.
Abstract
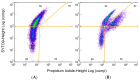
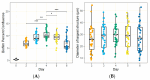
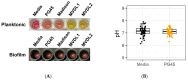
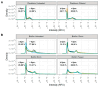
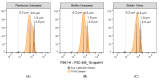
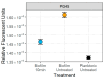
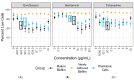
Mycoplasma spp. are facultative pathogens that contribute to the pathogenesis of multiple bovine diseases, including the bovine respiratory disease complex, and have been shown to form biofilms. Biofilm formation is associated with increased antibiotic resistance in many organisms, but accurate determination of antimicrobial susceptibility in biofilms is challenging. In Mycoplasma spp., antimicrobial susceptibility is routinely determined using metabolic pH-dependent color change. However, biofilm formation can lead to reduced metabolism, making interpretation of metabolic readouts difficult. Therefore, we developed and optimized a new flow cytometry-based method for antimicrobial susceptibility testing in biofilm-forming Mycoplasma, termed the live/dead antimicrobial susceptibility test (LD-AST). The LD-AST measures the proportion of live bacteria upon exposure to antibiotics, works robustly with both planktonic and biofilm cultures, and enables the determination of the minimum bactericidal concentration (MBC) for a given antibiotic. We used two strains of Mycoplasma bovis (Donetta PG45 and Madison) and two clinical Mycoplasma bovoculi isolates (MVDL1 and MVDL2) to determine the impact of biofilm growth on antimicrobial susceptibility for gentamicin, enrofloxacin, or tetracycline. All Mycoplasma strains were susceptible to all antibiotics when cultured as planktonic cells, with MBCs in the expected range. However, three out of four strains (Donetta PG45, MVDL1, and MVDL2) were completely resistant to all three antibiotics when newly adhered biofilms were analyzed, whereas M. bovis Madison gave variable results. For mature biofilms that were cultured for 4-5 days before antibiotic exposure, results also were variable, with some strains showing an increased resistance with certain antibiotics and a decreased resistance with others. Overall, these results are consistent with earlier reports that biofilms can exhibit increased antimicrobial resistance.
Keywords: Mycoplasma bovis; Mycoplasma sp.; antimicrobial resistance; assay development; biofilm formation; flow cytometry; live/dead staining.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Wilson D.J., Skirpstunas R.T., Trujillo J.D., Cavender K.B., Bagley C.V., Harding R.L. Unusual history and initial clinical signs of Mycoplasma bovis mastitis and arthritis in first-lactation cows in a closed commercial dairy herd. J. Am. Vet. Med. Assoc. 2007;230:1519–1523. doi: 10.2460/javma.230.10.1519. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

