Gram Negative Biofilms: Structural and Functional Responses to Destruction by Antibiotic-Loaded Mixed Polymeric Micelles
- PMID: 39770872
- PMCID: PMC11728461
- DOI: 10.3390/microorganisms12122670
Gram Negative Biofilms: Structural and Functional Responses to Destruction by Antibiotic-Loaded Mixed Polymeric Micelles
Abstract
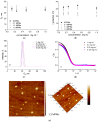
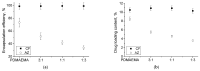
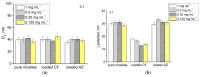
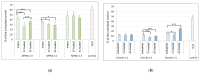
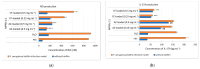
Biofilms are a well-known multifactorial virulence factor with a pivotal role in chronic bacterial infections. Their pathogenicity is determined by the combination of strain-specific mechanisms of virulence and the biofilm extracellular matrix (ECM) protecting the bacteria from the host immune defense and the action of antibacterials. The successful antibiofilm agents should combine antibacterial activity and good biocompatibility with the capacity to penetrate through the ECM. The objective of the study is the elaboration of biofilm-ECM-destructive drug delivery systems: mixed polymeric micelles (MPMs) based on a cationic poly(2-(dimethylamino)ethyl methacrylate)-b-poly(ε-caprolactone)-b-poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA35-b-PCL70-b-PDMAEMA35) and a non-ionic poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO100-b-PPO65-b-PEO100) triblock copolymers, loaded with ciprofloxacin or azithromycin. The MPMs were applied on 24 h pre-formed biofilms of Escherichia coli and Pseudomonas aeruginosa (laboratory strains and clinical isolates). The results showed that the MPMs were able to destruct the biofilms, and the viability experiments supported drug delivery. The biofilm response to the MPMs loaded with the two antibiotics revealed two distinct patterns of action. These were registered on the level of both bacterial cell-structural alterations (demonstrated by scanning electron microscopy) and the interaction with host tissues (ex vivo biofilm infection model on skin samples with tests on nitric oxide and interleukin (IL)-17A production).
Keywords: biocompatibility; biofilm destruction; cationic polymers; drug delivery; ex vivo skin model; extracellular vesicles; mixed polymer micelles; nanotubules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Damyanova T., Dimitrova P.D., Borisova D., Topouzova-Hristova T., Haladjova E., Paunova-Krasteva T. An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention. Pharmaceutics. 2024;16:162. doi: 10.3390/pharmaceutics16020162. - DOI - PMC - PubMed
-
- Stoitsova S., Paunova-Krasteva T., Dimitrova P.D., Damyanova T. The Concept for the Antivirulence Therapeutics Approach as Alternative to Antibiotics: Hope or Still a Fiction? Biotechnol. Biotechnol. Equip. 2022;36:697–705. doi: 10.1080/13102818.2022.2106887. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

