Lutein Maintains Bone Mass In Vitro and In Vivo Against Disuse-Induced Bone Loss in Hindlimb-Unloaded Mice
- PMID: 39770893
- PMCID: PMC11678298
- DOI: 10.3390/nu16244271
Lutein Maintains Bone Mass In Vitro and In Vivo Against Disuse-Induced Bone Loss in Hindlimb-Unloaded Mice
Abstract
Background: Lutein, a carotenoid, exhibits various biological activities such as maintaining the health of the eye, skin, heart, and bone. Recently, we found that lutein has dual roles in suppressing bone resorption and promoting bone formation. In this study, we examined the effects of lutein in a disuse-induced osteoporosis model using hindlimb-unloaded (HLU) mice.
Methods: Osteoclast differentiation was assessed by coculturing mouse primary osteoblasts and bone marrow cells or culturing a mouse osteoclast precursor cell line. The bone-resorbing activity was determined by mouse calvarial organ cultures. An in situ docking simulation was conducted to reveal the interaction of lutein and IκB kinase (IKK) β protein. HLU mice were fed a 1% lutein-containing diet for two weeks, and the femoral bone mass was measured by μCT.
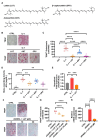
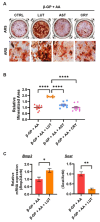
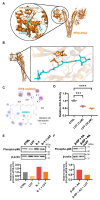
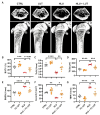
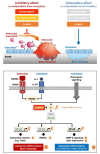
Results: Osteoclast differentiation is significantly inhibited by lutein, astaxanthin, and β-cryptoxanthin. In contrast, only lutein promoted osteoblastic calcified bone nodule formation. To elucidate the molecular role of lutein, we functionally analyzed the NF-κB complex, a molecule involved in bone metabolism, especially in osteoclasts. Docking simulations showed that lutein binds to IKK, thus inhibiting the activation of NF-κB. In a cell culture analysis, the phosphorylation of p65, the active form of NF-κB in osteoblasts, was suppressed by lutein treatment. In vivo, a μCT analysis of the bone microarchitecture showed that lutein improves several bone parameters while maintaining bone mass.
Conclusions: Lutein is effective in maintaining bone mass by controlling both bone resorption and formation, which is applied to prevent disuse-induced osteoporosis.
Keywords: astaxanthin; beta-cryptoxanthin; bone formation; bone resorption; disuse osteoporosis; lutein; osteoclast.
Conflict of interest statement
The authors declare no conflicts of interest in association with the present study.
Figures
References
-
- Inada M., Matsumoto C., Uematsu S., Akira S., Miyaura C. Membrane-Bound Prostaglandin E Synthase-1-Mediated Prostaglandin E2 Production by Osteoblast Plays a Critical Role in Lipopolysaccharide-Induced Bone Loss Associated with Inflammation. J. Immunol. 2006;177:1879–1885. doi: 10.4049/jimmunol.177.3.1879. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

