Caralluma fimbriata Extract Improves Vascular Dysfunction in Obese Mice Fed a High-Fat Diet
- PMID: 39770917
- PMCID: PMC11678847
- DOI: 10.3390/nu16244296
Caralluma fimbriata Extract Improves Vascular Dysfunction in Obese Mice Fed a High-Fat Diet
Abstract
Background: Obesity is a risk factor for developing cardiovascular diseases (CVDs) by impairing normal vascular function. Natural products are gaining momentum in the clinical setting due to their high efficacy and low toxicity. Caralluma fimbriata extract (CFE) has been shown to control appetite and promote weight loss; however, its effect on vascular function remains poorly understood. This study aimed to determine the effect that CFE had on weight loss and vascular function in mice fed a high-fat diet (HFD) to induce obesity, comparing this effect to that of lorcaserin (LOR) (an anti-obesity pharmaceutical) treatment.
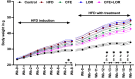
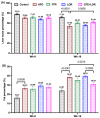
Methods: C57BL/6J male mice (n = 80) were fed a 16-week HFD to induce obesity prior to being treated with CFE and LOR as standalone treatments or in conjunction. Body composition data, such as weight gain and fat mass content were measured, isometric tension analyses were performed on isolated abdominal aortic rings to determine relaxation responses to acetylcholine, and immunohistochemistry studies were utilized to determine the expression profiles on endothelial nitric oxide synthase (eNOS) and cell stress markers (nitrotyrosine (NT) and 78 kDa glucose-regulated protein (GRP78)) in the endothelial, medial and adventitial layers of aortic rings.
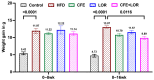
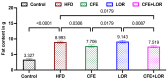
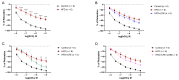
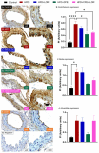
Results: The results demonstrated that CFE and CFE + LOR treatments significantly reduced weight gain (17%; 24%) and fat mass deposition (14%; 16%). A HFD markedly reduced acetylcholine-mediated relaxation (p < 0.05, p < 0.0001) and eNOS expression (p < 0.0001, p < 0.01) and significantly increased NT (p < 0.05, p < 0.0001) and GRP78 (p < 0.05, p < 0.01, p < 0.001). Obese mice treated with CFE exhibited significantly improved ACh-induced relaxation responses, increased eNOS (p < 0.05, p < 0.01) and reduced NT (p < 0.01) and GRP78 (p < 0.05, p < 0.01) expression.
Conclusions: Thus, CFE alone or in combination with LOR could serve as an alternative strategy for preventing obesity-related cardiovascular diseases.
Keywords: Caralluma fimbriata extract; bio-active compounds; high-fat diet; lorcaserin; natural product; obesity; vascular dysfunction.
Conflict of interest statement
The authors declare no conflicts of interest. A.Z. co-owns Zultek Engineering (Melbourne, VIC, Australia), the provider of product OB8 used for isometric tension studies. The authors declare that this study received funding from Gencor Pacific Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures


References
-
- Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation. 2022;145:e153–e639. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

