Transcriptional and Metabolic Changes Following Repeated Fasting and Refeeding of Adipose Stem Cells Highlight Adipose Tissue Resilience
- PMID: 39770930
- PMCID: PMC11676188
- DOI: 10.3390/nu16244310
Transcriptional and Metabolic Changes Following Repeated Fasting and Refeeding of Adipose Stem Cells Highlight Adipose Tissue Resilience
Abstract
Background: Obesity and related metabolic disorders have reached epidemic levels, calling for diverse therapeutic strategies. Altering nutrient intake, timing and quantity by intermittent fasting seems to elicit beneficial health effects by modulating endocrine and cell signaling networks. This study explores the impact of cyclic nutrient availability in the form of every-other-day fasting (EODF) on human adipose stem cells (ASCs).
Methods: We subjected ASCs to repeated fasting/refeeding (F/R) cycles, mimicking low glucose/high fatty acid (LGHF) conditions, and assessed phenotypic and transcriptomic changes, lipid storage capacity, insulin sensitivity, and differentiation potential.
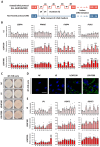
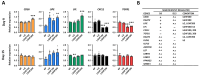
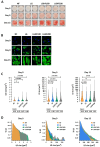
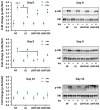
Results: Four consecutive F/R cycles induced significant changes in adipogenic gene expression, with upregulation of FABP4 and PLIN1 during fasting, and increased lipid storage in the ASCs. Upon differentiation, ASCs exposed to LGHF conditions retained a transient increase in lipid droplet size and altered fatty acid metabolism gene expression until day 9. However, these changes dissipated by day 15 of differentiation, suggesting a limited duration of fasting-induced transcriptional and adipogenic memory. Despite initial effects, ASCs showed resilience, returning to a physiological trajectory during differentiation, with respect to gene expression and lipid metabolism.
Conclusions: These findings suggest that the long-term effects of EODF on the ASC niche may be transient, emphasizing the ability of the adipose tissue to adapt and restore homeostasis.
Keywords: adipogenesis; intermittent fasting; metabolic memory; resilience.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Dynamic changes in chromatin accessibility, altered adipogenic gene expression, and total versus de novo fatty acid synthesis in subcutaneous adipose stem cells of normal-weight polycystic ovary syndrome (PCOS) women during adipogenesis: evidence of cellular programming.Clin Epigenetics. 2020 Nov 23;12(1):181. doi: 10.1186/s13148-020-00970-x. Clin Epigenetics. 2020. PMID: 33228780 Free PMC article.
-
The role of serum amyloid A1 in the adipogenic differentiation of human adipose-derived stem cells basing on single-cell RNA sequencing analysis.Stem Cell Res Ther. 2022 May 7;13(1):187. doi: 10.1186/s13287-022-02873-5. Stem Cell Res Ther. 2022. PMID: 35525990 Free PMC article.
-
Epigenetic reprogramming of H3K4me3 in adipose-derived stem cells by HFS diet consumption leads to a disturbed transcriptomic profile in adipocytes.Am J Physiol Endocrinol Metab. 2024 Jul 1;327(1):E13-E26. doi: 10.1152/ajpendo.00093.2024. Epub 2024 May 8. Am J Physiol Endocrinol Metab. 2024. PMID: 38717362
-
Adipose stem cells in obesity: challenges and opportunities.Biosci Rep. 2020 Jun 26;40(6):BSR20194076. doi: 10.1042/BSR20194076. Biosci Rep. 2020. PMID: 32452515 Free PMC article. Review.
-
Lipid Storage, Lipolysis, and Lipotoxicity in Obesity.Adv Exp Med Biol. 2024;1460:97-129. doi: 10.1007/978-3-031-63657-8_4. Adv Exp Med Biol. 2024. PMID: 39287850 Review.
References
-
- World Obesity Federation . World Obesity Atlas 2023. World Obesity Federation; London, UK: 2023. p. 232.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

