Curcumin and Its Potential to Target the Glycolytic Behavior of Lactate-Acclimated Prostate Carcinoma Cells with Docetaxel
- PMID: 39770959
- PMCID: PMC11677565
- DOI: 10.3390/nu16244338
Curcumin and Its Potential to Target the Glycolytic Behavior of Lactate-Acclimated Prostate Carcinoma Cells with Docetaxel
Abstract
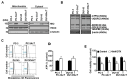
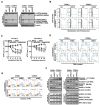
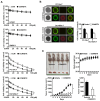
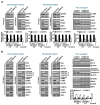
Background: Dysregulated cellular metabolism is known to be associated with drug resistance in cancer treatment. Methods: In this study, we investigated the impact of cellular adaptation to lactic acidosis on intracellular energy metabolism and sensitivity to docetaxel in prostate carcinoma (PC) cells. The effects of curcumin and the role of hexokinase 2 (HK2) in this process were also examined. Results: PC-3AcT and DU145AcT cells that preadapted to lactic acid displayed increased growth behavior, increased dependence on glycolysis, and reduced sensitivity to docetaxel compared to parental PC-3 and DU145 cells. Molecular analyses revealed activation of the c-Raf/MEK/ERK pathway, upregulation of cyclin D1, cyclin B1, and p-cdc2Thr161, and increased levels and activities of key regulatory enzymes in glycolysis, including HK2, in lactate-acclimated cells. HK2 knockdown resulted in decreased cell growth and glycolytic activity, decreased levels of complexes I-V in the mitochondrial electron transport chain, loss of mitochondrial membrane potential, and depletion of intracellular ATP, ultimately leading to cell death. In a xenograft animal model, curcumin combined with docetaxel reduced tumor size and weight, induced downregulation of glycolytic enzymes, and stimulated the upregulation of apoptotic and necroptotic proteins. This was consistent with the in vitro results from 2D monolayer and 3D spheroid cultures, suggesting that the efficacy of curcumin is not affected by docetaxel. Conclusions: Overall, our findings suggest that metabolic plasticity through enhanced glycolysis observed in lactate-acclimated PC cells may be one of the underlying causes of docetaxel resistance, and targeting glycolysis by curcumin may provide potential for drug development that could improve treatment outcomes in PC patients.
Keywords: apoptosis; chemoresistance; curcumin; glycolysis; lactic acid; necroptosis; prostate cancer cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
ERK1/2-Dependent Inhibition of Glycolysis in Curcumin-Induced Cytotoxicity of Prostate Carcinoma Cells.Biomed Res Int. 2022 Aug 24;2022:7626405. doi: 10.1155/2022/7626405. eCollection 2022. Biomed Res Int. 2022. PMID: 36060138 Free PMC article.
-
Curcumin Targets Both Apoptosis and Necroptosis in Acidity-Tolerant Prostate Carcinoma Cells.Biomed Res Int. 2021 May 22;2021:8859181. doi: 10.1155/2021/8859181. eCollection 2021. Biomed Res Int. 2021. PMID: 34095313 Free PMC article.
-
microRNA-323 upregulation promotes prostate cancer growth and docetaxel resistance by repressing p73.Biomed Pharmacother. 2018 Jan;97:528-534. doi: 10.1016/j.biopha.2017.10.040. Epub 2017 Nov 6. Biomed Pharmacother. 2018. PMID: 29091904
-
Arctigenin induces necroptosis through mitochondrial dysfunction with CCN1 upregulation in prostate cancer cells under lactic acidosis.Mol Cell Biochem. 2020 Apr;467(1-2):45-56. doi: 10.1007/s11010-020-03699-6. Epub 2020 Feb 17. Mol Cell Biochem. 2020. PMID: 32065351
-
miR-375 induces docetaxel resistance in prostate cancer by targeting SEC23A and YAP1.Mol Cancer. 2016 Nov 10;15(1):70. doi: 10.1186/s12943-016-0556-9. Mol Cancer. 2016. PMID: 27832783 Free PMC article.
Cited by
-
Roles and therapeutic potential of the SLC family in prostate cancer-literature review.BMC Urol. 2025 Feb 18;25(1):32. doi: 10.1186/s12894-025-01714-w. BMC Urol. 2025. PMID: 39966814 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

