Evaluating the Impact of Multimodal Prehabilitation with High Protein Oral Nutritional Supplementation (HP ONS) with Beta-Hydroxy Beta-Methylbutyrate (HMB) on Sarcopenic Surgical Patients-Interim Analysis of the HEROS Study
- PMID: 39770973
- PMCID: PMC11677323
- DOI: 10.3390/nu16244351
Evaluating the Impact of Multimodal Prehabilitation with High Protein Oral Nutritional Supplementation (HP ONS) with Beta-Hydroxy Beta-Methylbutyrate (HMB) on Sarcopenic Surgical Patients-Interim Analysis of the HEROS Study
Abstract
Background: Multimodal prehabilitation programs, which may incorporate nutritional supplementation and exercise, have been developed to combat sarcopenia in surgical patients to enhance post-operative outcomes. However, the optimal regime remains unknown. The use of beta-hydroxy beta-methylbutyrate (HMB) has beneficial effects on muscle mass and strength. However, its effect on muscle quality in the perioperative setting has yet to be established. This study aims to explore the impact of a multimodal prehabilitation program using a bundle of care that includes high-protein oral nutritional supplementation (HP ONS) with HMB and resistance exercise on muscle quality and functional outcomes in sarcopenic surgical patients.
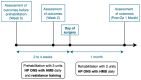
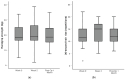
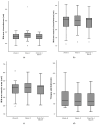
Methods: Sarcopenic adult patients undergoing elective major gastrointestinal surgeries were recruited for this pilot interventional cohort study. They were enrolled in a 2-4-week multimodal prehabilitation program comprising resistance exercise, nutritional supplementation, vitamin supplementation, comorbid optimization and smoking cessation. Participants were provided three units of HP ONS with HMB per day pre-operatively. The primary outcome was changes in intramuscular adipose tissue (IMAT) as a proxy of muscle quality, assessed using Artificial Intelligence (AI)-aided ultrasonography. Secondary outcomes include changes in anthropometric measurements and functional characteristics. Outcomes were measured before prehabilitation, after prehabilitation and 1 month post-operatively.
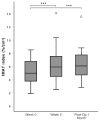
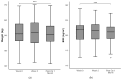
Results: A total of 36 sarcopenic patients, with a median age of 71.5 years, were included in this study. There was an increase in the IMAT index after two weeks of prehabilitation (p = 0.032) to 1 month after surgery (p = 0.028). Among functional parameters, improvement was observed in gait speed (p = 0.01) after two weeks of prehabilitation, which returned to baseline post-operatively. The median length of hospital stay was 7 (range: 2-75) days.
Conclusions: The increase in the IMAT index in a sarcopenic surgical cohort undergoing prehabilitation may be due to altered muscle metabolism in elderly sarcopenic patients. A prehabilitation regime in sarcopenic patients incorporating HP ONS with HMB and resistance exercise is feasible and is associated with increased gait speed.
Keywords: beta-hydroxy beta-methylbutyrate; intramuscular adipose tissue; oral nutritional supplementation; prehabilitation; sarcopenia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Koon-Yee Lee G., Chun-Ming Au P., Hoi-Yee Li G., Chan M., Li H.L., Man-Yung Cheung B., Chi-Kei Wong I., Ho-Fun Lee V., Mok J., Hon-Kei Yip B., et al. Sarcopenia and mortality in different clinical conditions: A meta-analysis. Osteoporos Sarcopenia. 2021;7((Suppl. S1)):S19–S27. doi: 10.1016/j.afos.2021.02.001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

