Dietary Modulation of the Immune System
- PMID: 39770983
- PMCID: PMC11676904
- DOI: 10.3390/nu16244363
Dietary Modulation of the Immune System
Abstract
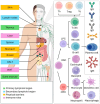
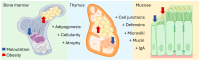
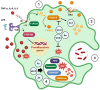
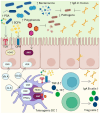
Recent insights into the influence of nutrition on immune system components have driven the development of dietary strategies targeting the prevention and management of major metabolic-inflammatory diseases. This review summarizes the bidirectional relationship between nutrition and immunocompetence, beginning with an overview of immune system components and their functions. It examines the effects of nutritional status, dietary patterns, and food bioactives on systemic inflammation, immune cell populations, and lymphoid tissues, as well as their associations with infectious and chronic disease pathogenesis. The mechanisms by which key nutrients influence immune constituents are delineated, focusing on vitamins A, D, E, C, and B, as well as minerals including zinc, iron, and selenium. Also highlighted are the immunomodulatory effects of polyunsaturated fatty acids as well as bioactive phenolic compounds and probiotics, given their expanding relevance. Each section addresses the implications of nutritional and nutraceutical interventions involving these nutrients within the broader context of major infectious, metabolic, and inflammatory diseases. This review further underscores that, while targeted nutrient supplementation can effectively restore immune function to optimal levels, caution is necessary in certain cases, as it may increase morbidity in specific diseases. In other instances, dietary counseling should be integrated to ensure that therapeutic goals are achieved safely and effectively.
Keywords: chronic diseases; immunity; infection; inflammation; nutrients.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Sirisinha S., Darip M.D., Moongkarndi P., Ongsakul M., Lamb A.J. Impaired local immune response in vitamin A-deficient rats. [(accessed on 16 July 2023)];Clin. Exp. Immunol. 1980 40:127. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC1536953/ - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

