Comparative Clinical Study on Magnesium Absorption and Side Effects After Oral Intake of Microencapsulated Magnesium (MAGSHAPETM Microcapsules) Versus Other Magnesium Sources
- PMID: 39770988
- PMCID: PMC11677548
- DOI: 10.3390/nu16244367
Comparative Clinical Study on Magnesium Absorption and Side Effects After Oral Intake of Microencapsulated Magnesium (MAGSHAPETM Microcapsules) Versus Other Magnesium Sources
Abstract
Background/objectives: Magnesium (Mg)-based food supplements contribute to the maintenance of adequate levels of Mg that are essential for overall health and well-being. The aim of this double-blind, randomized, cross-over clinical study was to assess the plasma Mg levels in volunteers following the oral administration of a magnesium-based nutraceutical ingredient, MAGSHAPETM microcapsules (Mg-MS), in comparison to other commonly used magnesium sources, including the following: Mg Oxide (MgO), Mg Citrate (Mg-C), and Mg bisglycinate (Mg-BG).
Methods: A total of 40 healthy women and men were put on a low-Mg diet for 7 days, and after 8 h of fasting, a blood sample was taken from a digital puncture before (0 h) and 1 h, 4 h, and 6 h after the oral intake of each product.
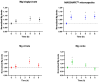
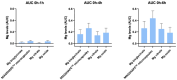
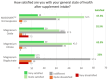
Results: Our results showed that the blood plasma levels of Mg increased significantly at all tested time-points after the oral intake of Mg-MS, while the blood plasma levels of Mg increased significantly only after 1 and 4 h of the oral intake of MgO and Mg-C, respectively. However, no significant increase in Mg levels was observed upon the intake of Mg-BG. Interestingly, the Mg-MS microencapsulation technology was observed to enable a sustained increase in plasma Mg levels over the duration of this study, i.e., 1, 4, and 6 h after oral intake. A direct comparison of the increase in plasma Mg levels over the 6 h period revealed that the Mg-MS microencapsulation technology significantly increased Mg bioavailability compared to the non-microencapsulated MgO. Our study also showed that, compared to the other Mg sources tested, the Mg-MS microencapsulation technology reduced adverse side effects commonly associated with Mg supplementation, specifically with regard to increased intestinal motility and sensations of gastric heaviness following oral administration.
Conclusions: Altogether, this clinical study introduced MAGSHAPETM microcapsules as a bioavailable and well-tolerated alternative to existing Mg-based ingredients used in food supplements.
Keywords: absorption; bioavailability; food supplement; magnesium; plasma; side effects; sustained release.
Conflict of interest statement
D.P., J.M.M. and J.L.M. were employed by Bionos Biotech SL. T.N. and A.C. were employed by Lubrizol Life Science. The authors declare that this study received funding from Lubrizol Life Science. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures



References
-
- Dietary Reference Values for Magnesium|EFSA [Internet] 2015. [(accessed on 25 June 2024)]. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4186.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

