Efficacy of Oral Probiotic Supplementation in Preventing Vulvovaginal Infections During Pregnancy: A Randomized and Placebo-Controlled Clinical Trial
- PMID: 39771026
- PMCID: PMC11676156
- DOI: 10.3390/nu16244406
Efficacy of Oral Probiotic Supplementation in Preventing Vulvovaginal Infections During Pregnancy: A Randomized and Placebo-Controlled Clinical Trial
Abstract
Background/objective: This study aimed to investigate the efficacy of oral probiotic supplementation in preventing vulvovaginal infections (VVIs) in pregnant women, specifically focusing on abnormal vaginal flora (AVF), bacterial vaginosis (BV), and vulvovaginal candidiasis (VVC).
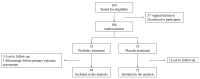
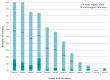
Methods: A multicenter-prospective-randomized, double-blind, placebo-controlled trial was conducted during 2016-2019. Women with normal vaginal flora (Nugent score < 4 and no candida) were divided into a research group, receiving 2 capsules/day of oral probiotic formula containing Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus, Lacticaseibacillus paracasei, Lacticaseibacillus rhamnosus, and Streptococcus thermophilus, or a control group, receiving a placebo until delivery. Once a month and following complaints, a vaginal smear was taken to assess vaginal flora. Vaginal colonization with the specific lactobacilli from the probiotic capsules was detected using the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. The primary outcome was the rate of women who developed VVI.
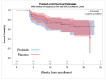
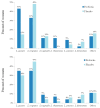
Results: Forty-nine and fifty-one women were analyzed in the probiotic and placebo cohorts, respectively. There was no difference in the rate of VVI between probiotic and placebo groups (14 (29%) versus 14 (27%), respectively; p = 0.80). No woman had vaginal colonization with lactobacilli from the probiotic capsule.
Conclusions: The tested oral probiotic product did not reduce the rate of VVI in pregnant women with normal vaginal flora.
Keywords: abnormal vaginal flora; bacterial vaginosis; pregnancy; prevention; probiotics; vulvovaginal candidiasis; vulvovaginal infections.
Conflict of interest statement
All the authors declare that they have no conflicts of interest.
Figures
References
-
- Carey J.C., Klebanoff M.A., Hauth J.C., Hillier S.L., Thom E.A., Ernest J.M., Heine R.P., Nugent R.P., Fischer M.L., Leveno K.J., et al. Metronidazole to Prevent Preterm Delivery in Pregnant Women with Asymptomatic Bacterial Vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N. Engl. J. Med. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

