Iodine Deficiency Exacerbates Thyroidal and Neurological Effects of Developmental Perchlorate Exposure in the Neonatal and Adult Rat
- PMID: 39771057
- PMCID: PMC11679215
- DOI: 10.3390/toxics12120842
Iodine Deficiency Exacerbates Thyroidal and Neurological Effects of Developmental Perchlorate Exposure in the Neonatal and Adult Rat
Abstract
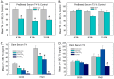
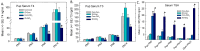
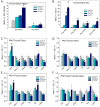
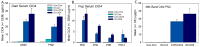
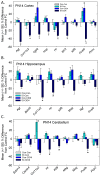
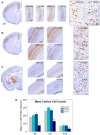
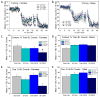
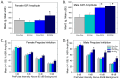
Thyroid hormones (THs) require iodine for biosynthesis and play critical roles in brain development. Perchlorate is an environmental contaminant that reduces serum THs by blocking the uptake of iodine from the blood to the thyroid gland. Using a pregnant rodent model, we examined the impact of maternal exposure to perchlorate under conditions of dietary iodine deficiency (ID) on the brain and behavior of offspring. We observed modest reductions in thyroxine (T4) in the serum of dams and no effect on T4 in pup serum in response to maternal exposure to 300 ppm of perchlorate in the drinking water. Likewise, serum T4 was reduced in ID dams, but, as with perchlorate, no effects were evident in the pup. However, when ID was coupled with perchlorate, reductions in pup serum THs and transcriptional alterations in the thyroid gland and pup brain were detected. These observations were accompanied by reductions in the number of cortical inhibitory interneurons containing the calcium-binding protein parvalbumin (Pvalb). Alterations in Pvalb expression in the neonatal brain were associated with deficits in the prepulse inhibition of acoustic startle in adult male offspring and enhanced fear conditioning in females. These findings support and extend structural defects in the brain previously reported in this model. Further, they underscore the critical need to consider additional non-chemical stressors in the determination of hazards and risks posed by environmental contaminants that affect the thyroid system.
Keywords: brain; development; iodine deficiency; neurotoxicity; perchlorate; thyroid hormone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Iodine supplementation for women during the preconception, pregnancy and postpartum period.Cochrane Database Syst Rev. 2017 Mar 5;3(3):CD011761. doi: 10.1002/14651858.CD011761.pub2. Cochrane Database Syst Rev. 2017. PMID: 28260263 Free PMC article.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
Epidural versus non-epidural or no analgesia for pain management in labour.Cochrane Database Syst Rev. 2018 May 21;5(5):CD000331. doi: 10.1002/14651858.CD000331.pub4. Cochrane Database Syst Rev. 2018. PMID: 29781504 Free PMC article.
-
Antibiotics and antiseptics for venous leg ulcers.Cochrane Database Syst Rev. 2013 Dec 23;(12):CD003557. doi: 10.1002/14651858.CD003557.pub4. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2014 Jan 10;(1):CD003557. doi: 10.1002/14651858.CD003557.pub5. PMID: 24363048 Updated.
References
-
- Zimmermann M.B. Chapter 25—Iodine and the iodine deficiency disorders. In: Marriott B.P., Birt D.F., Stalling V.A., Yates A.A., editors. Present Knowledge in Nutrition. 7th ed. Academic Press; Cambridge, MA, USA: 2020. pp. 429–441.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

