Modulation of Paracetamol-Induced Hepatotoxicity by Acute and Chronic Ethanol Consumption in Mice: A Study Pilot
- PMID: 39771072
- PMCID: PMC11679532
- DOI: 10.3390/toxics12120857
Modulation of Paracetamol-Induced Hepatotoxicity by Acute and Chronic Ethanol Consumption in Mice: A Study Pilot
Abstract
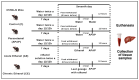
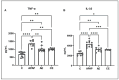
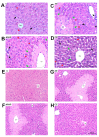
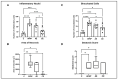
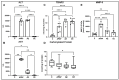
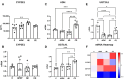
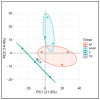
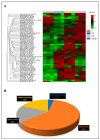
Paracetamol (APAP) overdose is the leading cause of drug-induced liver injury, leading to acute liver failure. However, the role of concurrent acute or chronic ethanol ingestion in this context requires further clarification. In this study, we investigated the effects of acute and chronic ethanol ingestion on APAP-induced hepatotoxicity. Male C57BL/6 mice were randomly allocated into four groups: control (C; water 2×/day for 7 days); APAP (single dose of APAP, 500 mg/kg); acute ethanol (AE; a single ethanol dose-10 mL/kg, and one hour later an overdose of APAP-500 mg/kg); chronic ethanol (CE; ethanol-10 mL/kg, 2×/day for 7 days; and on the last day, an overdose of APAP-500 mg/kg). The results showed that AE induced heightened liver damage, increased necrotic area, and elevated levels of ALT, AST, TBARS, and oxidized glutathione compared to the control group. The AE group exhibited diminished glutathione availability and elevated CYP2E1 levels compared to the other groups. CE maintained a hepatic profile similar to that of the control group in terms of necrosis index, ALT and AST levels, GSH/GSSG ratio, and CYP2E1 activity, along with the upregulation of gene expression of the glucuronidation enzyme compared to the APAP group. Proteomic analysis revealed that the AE protein profile closely resembled that of the APAP group, whereas the C and CE groups were clustered together. In conclusion, ethanol consumption differentially modulated APAP overdose-induced liver damage. Acute consumption exacerbated hepatotoxicity, similar to an APAP overdose alone, whereas chronic consumption appeared to mitigate this injury, at least within the parameters assessed in this study.
Keywords: acetaminophen; alcohol; ethanol; hepatotoxicity; liver damage; oxidative stress; paracetamol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Tittarelli R., Pellegrini M., Scarpellini M.G., Marinelli E., Bruti V., Di Luca N.M., Busardò F.P., Zaami S. Hepatotoxicity of paracetamol and related fatalities. Eur. Rev. Med. Pharmacol. Sci. 2017;21((Suppl. 1)):95–101. - PubMed
-
- Lv L., Ren S., Jiang H., Yan R., Chen W., Yan R., Dong J., Shao L., Yu Y. The oral administration of Lacticaseibacillus casei Shirota alleviates acetaminophen-induced liver injury through accelerated acetaminophen metabolism via the liver-gut axis in mice. mSphere. 2024;9:e0067223. doi: 10.1128/msphere.00672-23. - DOI - PMC - PubMed
-
- Bryan A., Pingali P., Faber A., Landry J., Akakpo J.Y., Jaeschke H., Li H., Lee W.S., May L., Patel B., et al. High dose acetaminophen with concurrent CYP2E1 inhibition has profound anti-cancer activity without liver toxicity. J. Pharmacol. Exp. Ther. 2024;388:209–217. doi: 10.1124/jpet.123.001772. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

