Multigenerational Consequences of Prenatal Exposure to Benzophenone-3 Demonstrate Sex- and Region-Dependent Neurotoxic and Pro-Apoptotic Effects in Mouse Brain
- PMID: 39771121
- PMCID: PMC11728767
- DOI: 10.3390/toxics12120906
Multigenerational Consequences of Prenatal Exposure to Benzophenone-3 Demonstrate Sex- and Region-Dependent Neurotoxic and Pro-Apoptotic Effects in Mouse Brain
Abstract
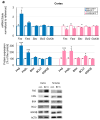
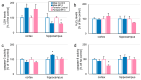
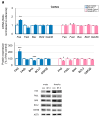
Benzophenone-3 (BP-3), commonly used as a UV filter in personal care products and as a stabilizer, is an alleged endocrine disruptor with potential neurodevelopmental impacts. Despite its abundance in the environment, the studies on its effect on brain development are scarce, especially in terms of multigenerational impact. In this work, for the first time, we examined neurotoxic and pro-apoptotic effects of BP-3 on mouse brain regions (cerebral cortex and hippocampus) in both the first (F1) and second (F2) generations after maternal exposure to environmentally relevant BP-3 levels. We found disregulated markers of cell damage (LDH, H2O2, caspase-3 and -8) and observed increased expression of pro-apoptotic Fas/FAS or Fasl/FASL. BP-3 exposure disrupted the BAX/BCL2 pathway, showing stronger effects in the F1 than in the F2 generation, with a dominance of extrinsic pathway (FAS, FASL, caspase-8) over intrinsic one (BAX, BCL2), suggesting that BP-3-induced apoptosis primarily operates via the extrinsic pathway and could impair brain homeostasis across generations. This study underscores the potential of BP-3 to increase multigenerational risks associated with disrupted neurodevelopment and highlights the importance of understanding its long-term neurotoxic effects.
Keywords: apoptosis; benzophenone-3; environmentally pervasive chemicals; multigenerational changes; neurotoxicity; prenatal exposure.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
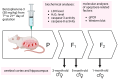
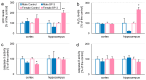
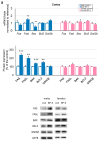

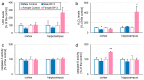


Similar articles
-
Prenatal exposure to benzophenone-3 (BP-3) induces apoptosis, disrupts estrogen receptor expression and alters the epigenetic status of mouse neurons.J Steroid Biochem Mol Biol. 2018 Sep;182:106-118. doi: 10.1016/j.jsbmb.2018.04.016. Epub 2018 Apr 25. J Steroid Biochem Mol Biol. 2018. PMID: 29704544
-
The effects of benzophenone-3 on apoptosis and the expression of sex hormone receptors in the frontal cortex and hippocampus of rats.Toxicol Lett. 2018 Oct 15;296:63-72. doi: 10.1016/j.toxlet.2018.08.006. Epub 2018 Aug 9. Toxicol Lett. 2018. PMID: 30099065
-
Effect of Combined Prenatal and Adult Benzophenone-3 Dermal Exposure on Factors Regulating Neurodegenerative Processes, Blood Hormone Levels, and Hematological Parameters in Female Rats.Neurotox Res. 2020 Mar;37(3):683-701. doi: 10.1007/s12640-020-00163-7. Epub 2020 Jan 23. Neurotox Res. 2020. PMID: 31970650 Free PMC article.
-
Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra.J Neuropathol Exp Neurol. 2003 Apr;62(4):329-39. doi: 10.1093/jnen/62.4.329. J Neuropathol Exp Neurol. 2003. PMID: 12722825 Review.
-
Is the commonly used UV filter benzophenone-3 a risk factor for the nervous system?Acta Biochim Pol. 2021 Aug 5;68(4):557-563. doi: 10.18388/abp.2020_5741. Acta Biochim Pol. 2021. PMID: 34351731 Review.
References
-
- Hasbani D.J., Ghaoui N., Haddad F., Shahla W.A., Saade S., Bandali T., Menhem Z., Saade D. Sun Protection Knowledge, Attitudes and Practices: A Lebanese-Based Survey. JEADV Clin. Pract. 2024;3:565–574. doi: 10.1002/jvc2.363. - DOI
-
- Afiouni R., Helou J., Bou-Orm I. Knowledge of the Risks of Ultraviolet Radiation, Sun Exposure Attitudes and Practices among Lebanese University Students. Prev. Med. Rep. 2024;47:102900. doi: 10.1016/j.pmedr.2024.102900. - DOI
-
- Sani M.A., Khezerlou A., Tavassoli M., Abedini A.H., McClements D.J. Development of Sustainable UV-Screening Food Packaging Materials: A Review of Recent Advances. Trends Food Sci. Technol. 2024;145:104366. doi: 10.1016/j.tifs.2024.104366. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

