Contamination Characteristics of Antibiotic Resistance Genes in Multi-Vector Environment in Typical Regional Fattening House
- PMID: 39771131
- PMCID: PMC11728509
- DOI: 10.3390/toxics12120916
Contamination Characteristics of Antibiotic Resistance Genes in Multi-Vector Environment in Typical Regional Fattening House
Abstract
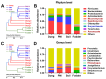
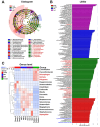
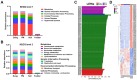
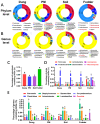
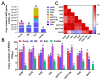
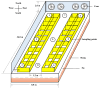
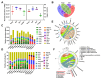
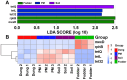
Antibiotic resistance genes (ARGs) are emerging as significant environmental contaminants, posing potential health risks worldwide. Intensive livestock farming, particularly swine production, is a primary contributor to the escalation of ARG pollution. In this study, we employed metagenomic sequencing and quantitative polymerase chain reaction to analyze the composition of microorganisms and ARGs across four vectors in a typical swine fattening facility: dung, soil, airborne particulate matter (PM), and fodder. Surprisingly, soil and PM harbored a higher abundance of microorganisms and ARGs than dung. At the same time, fodder was more likely to carry eukaryotes. Proteobacteria exhibited the highest propensity for carrying ARGs, with proportions 9-20 times greater than other microorganisms. Furthermore, a strong interrelation among various ARGs was observed, suggesting the potential for cooperative transmission mechanisms. These findings underscore the importance of recognizing soil and PM as significant reservoirs of ARGs in swine facilities alongside dung. Consequently, targeted measures should be implemented to mitigate their proliferation, mainly focusing on airborne PM, which can rapidly disseminate via air currents. Proteobacteria, given their remarkable carrying capacity for ARGs with the primary resistance mechanism of efflux, represent a promising avenue for developing novel control strategies against antibiotic resistance.
Keywords: antibiotics resistance gene; fattening house; metagenomics; multi-vector.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Dynamics of antibiotic resistance in poultry farms via multivector analysis.Poult Sci. 2025 Feb;104(2):104673. doi: 10.1016/j.psj.2024.104673. Epub 2024 Dec 15. Poult Sci. 2025. PMID: 39693773 Free PMC article.
-
Characteristics of and variation in airborne ARGs among urban hospitals and adjacent urban and suburban communities: A metagenomic approach.Environ Int. 2020 Jun;139:105625. doi: 10.1016/j.envint.2020.105625. Epub 2020 Apr 3. Environ Int. 2020. PMID: 32251897
-
Seasonal dynamics of the phage-bacterium linkage and associated antibiotic resistome in airborne PM2.5 of urban areas.Environ Int. 2024 Dec;194:109155. doi: 10.1016/j.envint.2024.109155. Epub 2024 Nov 17. Environ Int. 2024. PMID: 39647412
-
Research review on the pollution of antibiotic resistance genes in livestock and poultry farming environments.Ying Yong Sheng Tai Xue Bao. 2023 May;34(5):1415-1429. doi: 10.13287/j.1001-9332.202305.032. Ying Yong Sheng Tai Xue Bao. 2023. PMID: 37236960 Review. English.
-
Sources of Antibiotic Resistant Bacteria (ARB) and Antibiotic Resistance Genes (ARGs) in the Soil: A Review of the Spreading Mechanism and Human Health Risks.Rev Environ Contam Toxicol. 2021;256:121-153. doi: 10.1007/398_2020_60. Rev Environ Contam Toxicol. 2021. PMID: 33948742 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

