Combined Physiological and Transcriptomic Analyses of the Effects of Exogenous Trehalose on Salt Tolerance in Maize (Zea mays L.)
- PMID: 39771205
- PMCID: PMC11676066
- DOI: 10.3390/plants13243506
Combined Physiological and Transcriptomic Analyses of the Effects of Exogenous Trehalose on Salt Tolerance in Maize (Zea mays L.)
Abstract
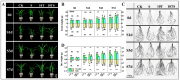
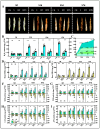
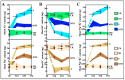
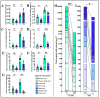
Soil salinization severely affects the quality and yield of maize. As a C4 plant with high efficiency in utilizing light and carbon dioxide, maize (Zea mays L.) is one of the most important crops worldwide. This study aims to investigate the pathways and mechanisms by which trehalose mediates the improvement of salt tolerance in maize through a combined analysis of physiology and transcriptomics. The results indicate that foliar application of trehalose treatment significantly increased maize biomass and antioxidant enzyme activity while reducing the H2O2 and Na+/K+ ratios in both the aerial and underground parts of the plant. Additionally, trehalose enhanced the total secretion of organic acids from maize roots, improving the soil microenvironment for maize growth under salt stress and alleviating Na+ toxicity. Transcriptomic data revealed that under salt stress, most differentially expressed genes (DEGs) were enriched in pathways related to photosynthesis, abscisic acid signaling, and sugar metabolism, and trehalose application increased the expression levels of these pathways, thereby mitigating the growth inhibition caused by salinity. This study elucidates mechanisms for enhancing salt tolerance in maize, providing theoretical support for improving its resilience and offering innovative strategies for utilizing a wide range of saline-alkali land.
Keywords: abscisic acid; antioxidant enzymes; maize (Zea mays L.); photosynthesis; salt stress; sugar metabolism; trehalose.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Rasheed A., Jie H.D., Ali B., He P.L., Zhao L., Ma Y.S., Xing H.C., Qari S.H., Hassan M.U., Hamid M.R., et al. Breeding Drought-Tolerant maize (Zea mays) using molecular breeding Tools: Recent advancements and future Prospective. Agronomy. 2023;13:1459. doi: 10.3390/agronomy13061459. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

