Gynoxys reinaldii Cuatrec. and Gynoxys pulchella (Kunth) Cass.: Chemical and Enantioselective Analyses of Two Unprecedented Essential Oils from Ecuador
- PMID: 39771242
- PMCID: PMC11677951
- DOI: 10.3390/plants13243543
Gynoxys reinaldii Cuatrec. and Gynoxys pulchella (Kunth) Cass.: Chemical and Enantioselective Analyses of Two Unprecedented Essential Oils from Ecuador
Abstract
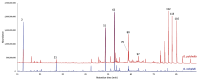
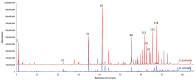
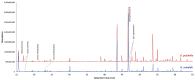
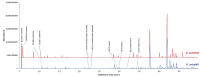
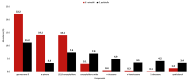
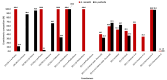
This study presents the first chemical and enantioselective analyses of essential oils (EOs) derived from the leaves of two endemic species, Gynoxys reinaldii Cuatrec. and Gynoxys pulchella (Kunth) Cass., from Loja, Ecuador. The distillation yields, by weight of dry plant material, were 0.04 ± 0.007% for G. reinaldii and 0.03 ± 0.002% for G. pulchella. For both plants, the chemical analyses were conducted by GC-MS (qualitative) and GC-FID (quantitative), on two stationary phases of different polarity (5% phenyl-methylpolysiloxane and polyethylene glycol). The major components of G. reinaldii EO included germacrene D (22.3-22.1%), α-pinene (14.2-14.1%), and (E)-β-caryophyllene (13.6-14.5%). Similarly, G. pulchella EO was characterized by germacrene D (9.5-12.9%), caryophyllene oxide (7.2-6.7%), and n-tricosane (4.9% in both columns). The enantioselective analyses were carried out with two columns, based on 2,3-diacetyl-6-tert-butyldimethylsilyl-β-cyclodextrin and 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin, detecting nine chiral terpenes and terpenoids. In G. reinaldii EO, (1S,5S)-(-)-α-pinene, (1S,5S)-(-)-β-pinene, (1S,5S)-(-)-sabinene, (R)-(-)-α-phellandrene, and (R)-(-)-β-phellandrene were enantiomerically pure, whereas cis-linalool oxide, linalool, terpinene-4-ol, and germacrene D were non-racemic mixtures of enantiomers. In G. pulchella, only (R)-(-)-α-phellandrene was enantiomerically pure. The detection of enantiomerically pure compounds may provide insights into the biosynthetic pathways and potential bioactivities of these EOs.
Keywords: asteraceae; enantiomeric composition; mass spectrometry; sesquiterpene; β-cyclodextrin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
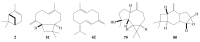
References
-
- Huxtable R.J., Schwarz S.K. The isolation of morphine—First principles in science and ethics. Mol. Interv. 2001;1:189–191. - PubMed
-
- Megadiverse Countries, UNEP-WCMC. [(accessed on 12 June 2024)]. Available online: https://www.biodiversitya-z.org/content/megadiverse-countries.
LinkOut - more resources
Full Text Sources
Miscellaneous

