Modeling Floral Induction in the Narrow-Leafed Lupin Lupinus angustifolius Under Different Environmental Conditions
- PMID: 39771246
- PMCID: PMC11678331
- DOI: 10.3390/plants13243548
Modeling Floral Induction in the Narrow-Leafed Lupin Lupinus angustifolius Under Different Environmental Conditions
Abstract
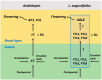
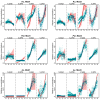
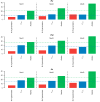
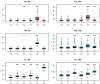
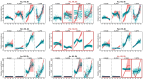
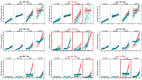
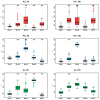
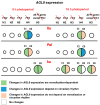
Flowering is initiated in response to environmental cues, with the photoperiod and ambient temperature being the main ones. The regulatory pathways underlying floral transition are well studied in Arabidopsis thaliana but remain largely unknown in legumes. Here, we first applied an in silico approach to infer the regulatory inputs of four FT-like genes of the narrow-leafed lupin Lupinus angustifolius. We studied the roles of FTc1, FTc2, FTa1, and FTa2 in the activation of meristem identity gene AGL8 in response to 8 h and 16 h photoperiods, vernalization, and the circadian rhythm. We developed a set of regression models of AGL8 regulation by the FT-like genes and fitted these models to the recently published gene expression data. The importance of the input from each FT-like gene or their combinations was estimated by comparing the performance of models with one or few FT-like genes turned off, thereby simulating loss-of-function mutations that were yet unavailable in L. angustifolius. Our results suggested that in the early flowering Ku line and intermediate Pal line, the FTc1 gene played a major role in floral transition; however, it acted through different mechanisms under short and long days. Turning off the regulatory input of FTc1 resulted in substantial changes in AGL8 expression associated with vernalization sensitivity and the circadian rhythm. In the wild ku line, we found that both FTc1 and FTa1 genes had an essential role under long days, which was associated with the vernalization response. These results could be applied both for setting up new experiments and for data analysis using the proposed modeling approach.
Keywords: FT-like genes; Lupinus angustifolius; floral induction; gene expression; legumes; mathematical modeling; meristem identity genes; narrow-leafed lupin; photoperiod; vernalization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Photoperiod and Vernalization Control of Flowering-Related Genes: A Case Study of the Narrow-Leafed Lupin (Lupinus angustifolius L.).Front Plant Sci. 2020 Oct 26;11:572135. doi: 10.3389/fpls.2020.572135. eCollection 2020. Front Plant Sci. 2020. PMID: 33193508 Free PMC article.
-
The loss of vernalization requirement in narrow-leafed lupin is associated with a deletion in the promoter and de-repressed expression of a Flowering Locus T (FT) homologue.New Phytol. 2017 Jan;213(1):220-232. doi: 10.1111/nph.14094. Epub 2016 Jul 15. New Phytol. 2017. PMID: 27418400
-
INDEL variation in the regulatory region of the major flowering time gene LanFTc1 is associated with vernalization response and flowering time in narrow-leafed lupin (Lupinus angustifolius L.).Plant Cell Environ. 2019 Jan;42(1):174-187. doi: 10.1111/pce.13320. Epub 2018 May 23. Plant Cell Environ. 2019. PMID: 29677403 Free PMC article.
-
Mechanisms of Vernalization-Induced Flowering in Legumes.Int J Mol Sci. 2022 Aug 31;23(17):9889. doi: 10.3390/ijms23179889. Int J Mol Sci. 2022. PMID: 36077286 Free PMC article. Review.
-
Nutritional composition and biological activity of narrow-leafed lupins (Lupinus angustifolius L.) hydrolysates and seeds.Food Chem. 2023 Sep 15;420:136104. doi: 10.1016/j.foodchem.2023.136104. Epub 2023 Apr 3. Food Chem. 2023. PMID: 37059020 Review.
References
LinkOut - more resources
Full Text Sources

