Virus-like Particles Produced in Plants: A Promising Platform for Recombinant Vaccine Development
- PMID: 39771262
- PMCID: PMC11678810
- DOI: 10.3390/plants13243564
Virus-like Particles Produced in Plants: A Promising Platform for Recombinant Vaccine Development
Abstract
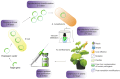
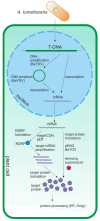
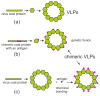
The capsid proteins of many viruses are capable of spontaneous self-assembly into virus-like particles (VLPs), which do not contain the viral genome and are therefore not infectious. VLPs are structurally similar to their parent viruses and are therefore effectively recognized by the immune system and can induce strong humoral and cellular immune responses. The structural features of VLPs make them an attractive platform for the development of potential vaccines and diagnostic tools. Chimeric VLPs can be obtained by attaching foreign peptides to capsid proteins. Chimeric VLPs present multiple copies of the antigen on their surface, thereby increasing the effectiveness of the immune response. Recombinant VLPs can be produced in different expression systems. Plants are promising biofactories for the production of recombinant proteins, including VLPs. The main advantages of plant expression systems are the overall low cost and safety of plant-produced products due to the absence of pathogens common to plants and animals. This review provides an overview of the VLP platform as an approach to developing plant-produced vaccines, focusing on the use of transient expression systems.
Keywords: biofactory; plant; transient expression; vaccine; virus-like particle.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Plant-produced chimeric virus-like particles - a new generation vaccine against African horse sickness.BMC Vet Res. 2019 Dec 3;15(1):432. doi: 10.1186/s12917-019-2184-2. BMC Vet Res. 2019. PMID: 31796116 Free PMC article.
-
Plant-derived virus-like particles as vaccines.Hum Vaccin Immunother. 2013 Jan;9(1):26-49. doi: 10.4161/hv.22218. Epub 2012 Sep 20. Hum Vaccin Immunother. 2013. PMID: 22995837 Free PMC article. Review.
-
Structural basis for the development of avian virus capsids that display influenza virus proteins and induce protective immunity.J Virol. 2015 Mar;89(5):2563-74. doi: 10.1128/JVI.03025-14. Epub 2014 Dec 17. J Virol. 2015. PMID: 25520499 Free PMC article.
-
Design of Novel Vaccines Based on Virus-Like Particles.Subcell Biochem. 2024;105:785-821. doi: 10.1007/978-3-031-65187-8_21. Subcell Biochem. 2024. PMID: 39738963 Review.
-
Virus-like Particle Vaccines and Platforms for Vaccine Development.Viruses. 2023 May 2;15(5):1109. doi: 10.3390/v15051109. Viruses. 2023. PMID: 37243195 Free PMC article. Review.
Cited by
-
Overcoming dengue vaccine challenges through next-generation virus-like particle immunization strategies.Front Cell Infect Microbiol. 2025 Jun 16;15:1614805. doi: 10.3389/fcimb.2025.1614805. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40589870 Free PMC article. Review.
-
Molecular Farming for Immunization: Current Advances and Future Prospects in Plant-Produced Vaccines.Vaccines (Basel). 2025 Feb 15;13(2):191. doi: 10.3390/vaccines13020191. Vaccines (Basel). 2025. PMID: 40006737 Free PMC article. Review.
-
Harnessing Transient Expression Systems with Plant Viral Vectors for the Production of Biopharmaceuticals in Nicotiana benthamiana.Int J Mol Sci. 2025 Jun 9;26(12):5510. doi: 10.3390/ijms26125510. Int J Mol Sci. 2025. PMID: 40564973 Free PMC article. Review.
-
The 6-kilodalton peptide 1 of the family Potyviridae: small in size but powerful in function.Front Microbiol. 2025 Jun 5;16:1605199. doi: 10.3389/fmicb.2025.1605199. eCollection 2025. Front Microbiol. 2025. PMID: 40539110 Free PMC article. Review.
References
-
- Pumpens P., Pushko P. Virus-like Particles: A Comprehensive Guide. 1st ed. Taylor & Francis Group; Milton, ON, Canada: 2022. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

