The Ameliorative Effect of Coumarin on Copper Toxicity in Citrus sinensis: Insights from Growth, Nutrient Uptake, Oxidative Damage, and Photosynthetic Performance
- PMID: 39771282
- PMCID: PMC11678025
- DOI: 10.3390/plants13243584
The Ameliorative Effect of Coumarin on Copper Toxicity in Citrus sinensis: Insights from Growth, Nutrient Uptake, Oxidative Damage, and Photosynthetic Performance
Abstract
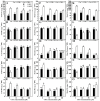
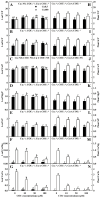
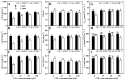
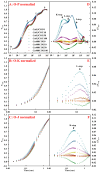
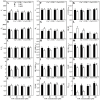
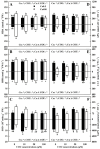
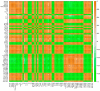
Excessive copper (Cu) has become a common physiological disorder restricting the sustainable production of citrus. Coumarin (COU) is a hydroxycinnamic acid that can protect plants from heavy metal toxicity. No data to date are available on the ameliorative effect of COU on plant Cu toxicity. 'Xuegan' (Citrus sinensis (L.) Osbeck) seedlings were treated for 24 weeks with nutrient solution containing two Cu levels (0.5 (Cu0.5) and 400 (Cu400) μM CuCl2) × four COU levels (0 (COU0), 10 (COU10), 50 (COU50), and 100 (COU100) μM COU). There were eight treatments in total. COU supply alleviated Cu400-induced increase in Cu absorption and oxidative injury in roots and leaves, decrease in growth, nutrient uptake, and leaf pigment concentrations and CO2 assimilation (ACO2), and photo-inhibitory impairment to the whole photosynthetic electron transport chain (PETC) in leaves, as revealed by chlorophyll a fluorescence (OJIP) transient. Further analysis suggested that the COU-mediated improvement of nutrient status (decreased competition of Cu2+ with Mg2+ and Fe2+, increased uptake of nutrients, and elevated ability to maintain nutrient balance) and mitigation of oxidative damage (decreased formation of reactive oxygen species and efficient detoxification system in leaves and roots) might lower the damage of Cu400 to roots and leaves (chloroplast ultrastructure and PETC), thereby improving the leaf pigment levels, ACO2, and growth of Cu400-treated seedlings.
Keywords: CO2 assimilation; chlorophyll a fluorescence (OJIP) transient; nutrient balance; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Boron-mediated amelioration of copper-toxicity in sweet orange [Citrus sinensis (L.) Osbeck cv. Xuegan] seedlings involved reduced damage to roots and improved nutrition and water status.Ecotoxicol Environ Saf. 2022 Apr 1;234:113423. doi: 10.1016/j.ecoenv.2022.113423. Epub 2022 Mar 17. Ecotoxicol Environ Saf. 2022. PMID: 35307619
-
Excess copper effects on growth, uptake of water and nutrients, carbohydrates, and PSII photochemistry revealed by OJIP transients in Citrus seedlings.Environ Sci Pollut Res Int. 2019 Oct;26(29):30188-30205. doi: 10.1007/s11356-019-06170-2. Epub 2019 Aug 17. Environ Sci Pollut Res Int. 2019. PMID: 31422532
-
Exogenous coumarin improves cell wall and plasma membrane stability and function by maintaining copper and calcium homeostasis in citrus roots under copper excess.Plant Physiol Biochem. 2025 Jul;224:109949. doi: 10.1016/j.plaphy.2025.109949. Epub 2025 Apr 29. Plant Physiol Biochem. 2025. PMID: 40319587
-
Effects of Low pH on Photosynthesis, Related Physiological Parameters, and Nutrient Profiles of Citrus.Front Plant Sci. 2017 Feb 21;8:185. doi: 10.3389/fpls.2017.00185. eCollection 2017. Front Plant Sci. 2017. PMID: 28270819 Free PMC article.
-
Raising pH Reduces Manganese Toxicity in Citrus grandis (L.) Osbeck by Efficient Maintenance of Nutrient Homeostasis to Enhance Photosynthesis and Growth.Plants (Basel). 2025 Aug 2;14(15):2390. doi: 10.3390/plants14152390. Plants (Basel). 2025. PMID: 40805740 Free PMC article.
References
-
- Broadley M., Brown P., Cakmak I., Rengel Z., Zhao F. Function of nutrients: Micronutrients. In: Marschner P., editor. Marschner’s Mineral Nutrition of Higher Plants. 3rd ed. Academic; Amsterdam, The Netherlands: 2012. pp. 191–248.
-
- Chen X.-F., Hua D., Zheng Z.-C., Zhang J., Huang W.-T., Chen H.-H., Huang Z.-R., Yang L.-T., Ye X., Chen L.-S. Boron-mediated amelioration of copper-toxicity in sweet orange [Citrus sinensis (L.) Osbeck cv. Xuegan] seedlings involved reduced damage to roots and improved nutrition and water status. Ecotoxicol. Environ. Saf. 2022;234:113423. doi: 10.1016/j.ecoenv.2022.113423. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

