Iron Deficiency in Tomatoes Reversed by Pseudomonas Strains: A Synergistic Role of Siderophores and Plant Gene Activation
- PMID: 39771283
- PMCID: PMC11677312
- DOI: 10.3390/plants13243585
Iron Deficiency in Tomatoes Reversed by Pseudomonas Strains: A Synergistic Role of Siderophores and Plant Gene Activation
Abstract
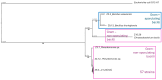
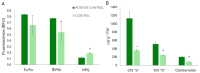
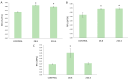
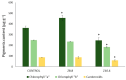
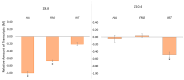
An alkaline pH in soils reduces Fe availability, limiting Fe uptake, compromising plant growth, and showing chlorosis due to a decrease in chlorophyll content. To achieve proper Fe homeostasis, dicotyledonous plants activate a battery of strategies involving not only Fe absorption mechanisms, but also releasing phyto-siderophores and recruiting siderophore-producing bacterial strains. A screening for siderophore-producing bacterial isolates from the rhizosphere of Pinus pinea was carried out, resulting in two Pseudomonas strains, Z8.8 and Z10.4, with an outstanding in vitro potential to solubilize Fe, Mn, and Co. The delivery of each strain to 4-week-old iron-starved tomatoes reverted chlorosis, consistent with enhanced Fe contents up to 40%. Photosynthesis performance was improved, revealing different strategies. While Z8.8 increased energy absorption together with enhanced chlorophyll "a" content, followed by enhanced energy dissipation, Z10.4 lowered pigment contents, indicating a better use of absorbed energy, leading to a better survival rate. The systemic reprogramming induced by both strains reveals a lower expression of Fe uptake-related genes, suggesting that both strains have activated plant metabolism to accelerate Fe absorption faster than controls, consistent with increased Fe content in leaves (47% by Z8.8 and 42% by Z10.4), with the difference probably due to the ability of Z8.8 to produce auxins affecting root structure. In view of these results, both strains are effective candidates to develop biofertilizers.
Keywords: Plant Growth Promoting Bacteria (PGPB); Pseudomonas; chlorosis reversion; iron nutrition; photosynthesis; siderophores.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
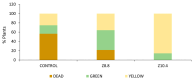
References
LinkOut - more resources
Full Text Sources

