Cryopreservation Protocols and the Associated Ultrastructural Changes in Dormant Buds of Vitis amurensis
- PMID: 39771288
- PMCID: PMC11679854
- DOI: 10.3390/plants13243590
Cryopreservation Protocols and the Associated Ultrastructural Changes in Dormant Buds of Vitis amurensis
Abstract
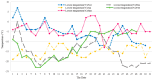
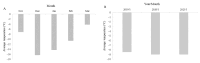
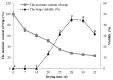
There is an urgent need for the cryopreservation of dormant buds to conserve the genetic resources of woody plants, particularly fruit trees, as this method is less time-consuming and relatively inexpensive. In the present study, three different cryopreservation protocols were tested on dormant buds from three varieties of Vitis amurensis Rupr. The explants were collected between November 2017 and March 2018. Twig segments harvested from field-grown plants, each containing one dormant bud, were desiccated in a low-temperature test chamber at -5 °C. The viability of the buds was highest (45%) after 28-30 days of desiccation, when the moisture content was approximately 25-30%. Cryopreservation using the CP3 protocol (which involves decreasing the temperature at a rate of 0.1 °C/min to -30 °C and holding this temperature for 24 h, followed by a 0.5 °C/min decline to -80 °C, a 1 °C/min decline to -180 °C, and finally reaching -196 °C in a CryoMed controlled rate freezer) significantly enhanced the viability (66.67%) when the samples were packed in aluminum-foil bags. Additionally, immersing the twigs in ice-cold (4 °C) water for 24 h in a refrigerator during thawing proved to be more conducive to viability. The dormant buds of all three V. amurensis varieties collected in January exhibited the highest viability after cryopreservation, followed by those collected in February and December. In contrast, the dormant buds collected in November and March showed the lowest viability after cryopreservation. The average viability of twigs of 'Shuanghong', 'Zuoshanyi', and 'Shuangfeng' collected between 2019 and 2021 all exceeded 60%. After the cryopreservation process, the outer multilayered cells in the buds were completely damaged; however, the inner cells exhibited moderate damage and were able to resume growth after thawing. Therefore, based on graft viability and histological observations, the dormant bud cryopreservation protocols tested in this study could be applicable to these three V. amurensis varieties.
Keywords: Vitis amurensis; conservation of resources; dormant buds; structural changes; viability.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures



Similar articles
-
Twig pre-harvest temperature significantly influences effective cryopreservation of Vaccinium dormant buds.Cryobiology. 2017 Feb;74:154-159. doi: 10.1016/j.cryobiol.2016.10.007. Epub 2016 Nov 10. Cryobiology. 2017. PMID: 27840093
-
Dormant bud cryopreservation of Rubus idaeus L.Cryobiology. 2025 Jun;119:105240. doi: 10.1016/j.cryobiol.2025.105240. Epub 2025 Mar 29. Cryobiology. 2025. PMID: 40158297
-
Successful Cryopreservation of Dormant Buds of Blackcurrant (Ribes nigrum L.) by Using Greenhouse-Grown Plants and In Vitro Recovery.Plants (Basel). 2021 Jul 10;10(7):1414. doi: 10.3390/plants10071414. Plants (Basel). 2021. PMID: 34371617 Free PMC article.
-
Recent advances in the cryopreservation of shoot-derived germplasm of economically important fruit trees of Actinidia, Diospyros, Malus, Olea, Prunus, Pyrus and Vitis.Biotechnol Adv. 2013 Mar-Apr;31(2):175-85. doi: 10.1016/j.biotechadv.2012.09.004. Epub 2012 Sep 27. Biotechnol Adv. 2013. PMID: 23022736 Review.
-
Indian cryogenebank conserving diverse plant genetic resources for the last three decades: Achievements and way forward.Cryobiology. 2025 Mar;118:105205. doi: 10.1016/j.cryobiol.2025.105205. Epub 2025 Jan 31. Cryobiology. 2025. PMID: 39870154 Review.
References
-
- Engelmann F., Engels J.M.M. Technologies and strategies for ex situ conservation; Proceedings of the Managing Plant Genetic Diversity, Proceedings of an International Conference; Kuala Lumpur, Malaysia. 12–16 June 2000; pp. 89–103. - DOI
-
- Lambardi M., De Carlo A., Benelli C., Bartolini G. Cryopreservation of woody species by vitrification of shoot tips and embryogenic tissue. Acta Hortic. 2001;560:125–128. doi: 10.17660/ActaHortic.2001.560.18. - DOI
-
- Reed B.M. Implementing cryopreservation for long-term germplasm preservation in vegetatively propagated species. In: Towill L.E., Bajaj Y.P.S., editors. Biotechnology in Agriculture and Forestry. Volume 50. Springer; Berlin/Heidelberg, Germany: 2002. pp. 22–33. Cryopreservation of Plant Germplasm II. - DOI
-
- Hao Y.-J., Deng X.-X. Genetically stable regeneration of apple plants from slow growth. Plant Cell Tissue Organ Cult. (PCTOC) 2003;72:253–260. doi: 10.1023/A:1022388728497. - DOI
-
- Höfer M. Conservation strategy of genetic resources in strawberry in Germany. Acta Hortic. 2011;918:139–146. doi: 10.17660/ActaHortic.2011.918.16. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

