Effects of Exogenous Spermidine on Seed Germination and Physiological Metabolism of Rice Under NaCl Stress
- PMID: 39771298
- PMCID: PMC11679135
- DOI: 10.3390/plants13243599
Effects of Exogenous Spermidine on Seed Germination and Physiological Metabolism of Rice Under NaCl Stress
Abstract
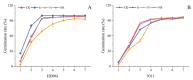
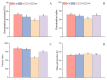
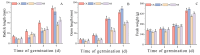
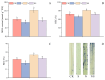
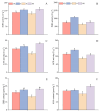
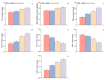
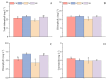
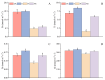
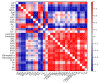
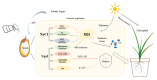
Salt stress is one of the principal abiotic stresses limiting agricultural production and seriously inhibiting seed germination rates. This study selected the salt-tolerant rice variety HD961 and the salt-sensitive rice variety 9311 as experimental materials to investigate the physiological and metabolic effects of exogenous Spd seed priming on rice seeds and seedlings under NaCl stress. The experiment involved treating rice seeds with 0.1 mmol·L-1 Spd and then subjecting them to 100 mmol·L-1 NaCl stress for 24 h, with sampling for analysis at the 24 h and the four-leaf-one-heart stage. The results indicated that under NaCl stress, the rice's germination and vigor indices significantly decreased. However, exogenous Spd seed priming reduced the accumulation of malondialdehyde, enhanced the capacity for osmotic adjustment, and increased the amylase and antioxidant activity by 50.07% and 26.26%, respectively. Under NaCl stress, the morphological development of rice seedlings was markedly inhibited, whereas exogenous Spd seed priming improved the aboveground and belowground biomass of the rice under stress conditions, as well as the content of photosynthetic pigments. It also reduced the damage to seedlings from electrical conductivity, helped maintain ionic balance, and promoted the excretion of Na+ and Cl- and the absorption of K+ and Ca2+. In the salt-sensitive rice variety 9311, the soluble protein content increased by 15.12% compared to the salt-tolerant rice variety HD961, especially under 100 mmol·L-1 NaCl stress, when the effect of exogenous Spd seed priming was more pronounced. In summary, these findings might provide new research perspectives and strategies for improving the salt tolerance of rice under NaCl stress.
Keywords: NaCl stress; rice; seed germination; seedling growth; spermidine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
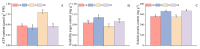
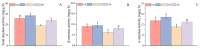
Similar articles
-
[Effect of exogenous Ca2+, ALA, SA and Spd on seed germination and physiological characteristics of Perilla frutescens seedlings under NaCl stress].Zhongguo Zhong Yao Za Zhi. 2010 Dec;35(24):3260-5. Zhongguo Zhong Yao Za Zhi. 2010. PMID: 21438385 Chinese.
-
[Effects of Exogenous Spermidine on Seed Germination and As Uptake and Accumulation of Rice Under As5+ Stress].Huan Jing Ke Xue. 2020 Mar 8;41(3):1505-1512. doi: 10.13227/j.hjkx.201909083. Huan Jing Ke Xue. 2020. PMID: 32608655 Chinese.
-
Spermidine or spermine pretreatment regulates organic metabolites and ions homeostasis in favor of white clover seed germination against salt toxicity.Plant Physiol Biochem. 2024 Feb;207:108379. doi: 10.1016/j.plaphy.2024.108379. Epub 2024 Jan 20. Plant Physiol Biochem. 2024. PMID: 38266560
-
Exogenous SA Affects Rice Seed Germination under Salt Stress by Regulating Na+/K+ Balance and Endogenous GAs and ABA Homeostasis.Int J Mol Sci. 2022 Mar 18;23(6):3293. doi: 10.3390/ijms23063293. Int J Mol Sci. 2022. PMID: 35328712 Free PMC article.
-
Seed priming to alleviate salinity stress in germinating seeds.J Plant Physiol. 2016 Mar 15;192:38-46. doi: 10.1016/j.jplph.2015.12.011. Epub 2016 Jan 16. J Plant Physiol. 2016. PMID: 26812088 Review.
References
-
- Chenyin P., Yu W., Fenghou S., Yongbao S. Review of the current research progress of seed germination inhibitors. Horticulturae. 2023;9:462. doi: 10.3390/horticulturae9040462. - DOI
-
- Rosińska A., Andrzejak R., Kakkerla V. Effect of osmopriming with melatonin on germination, vigor and health of Daucus carota L. seeds. Agriculture. 2023;13:749. doi: 10.3390/agriculture13040749. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

