Effect of Side Substituent on Comb-like Polysiloxane Membrane Pervaporation Properties During Recovery of Alcohols C2-C4 from Water
- PMID: 39771382
- PMCID: PMC11678792
- DOI: 10.3390/polym16243530
Effect of Side Substituent on Comb-like Polysiloxane Membrane Pervaporation Properties During Recovery of Alcohols C2-C4 from Water
Abstract
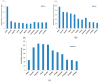
The pervaporation properties of membranes based on comb-like polysiloxanes when C2-C4 alcohols are removed from water were studied for the first time. It was established that membranes based on comb-like polysiloxanes with linear aliphatic and organosilicon substituents have increased permeability selectivity for C3+ alcohols. The obtained results were interpreted from the point of view of the solubility of the components of the separated mixture in polysiloxanes. It was shown that membranes based on polysiloxanes with linear substituents have increased butanol/water permeability selectivity (2.5-3.7). The achieved selectivity values correspond to the level of highly selective zeolite membranes, which allows for a reduction in energy consumption for the pervaporation removal of butanol by more than two times.
Keywords: alcohol recovery; comb-like polysiloxane; pervaporation; polymeric membrane; water treatment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- De Klerk A. Fischer–Tropsch Refining. John Wiley & Sons; Hoboken, NJ, USA: 2012. 619p
-
- Krylova A.Y., Kryazhev Y.G., Kulikova M.V., Kurkin V.I., Lyadov A.S., Sagitov S.A. Synthesis of Alcohols from CO and H2 on Iron Catalysts Containing Carbon Fiber. Solid Fuel Chem. 2011;45:322–326. doi: 10.3103/S036152191105003X. - DOI
-
- Krylova A.Y., Kurkin V.I., Kulikova M.V., Lyadov A.S., Sagitov S.A. Synthesis of Monohydric Alcohols from CO and H2 on Fe/Sibunit Catalysts. Solid Fuel Chem. 2011;45:281–285. doi: 10.3103/S0361521911040069. - DOI
-
- Kölbel H., Ralek M. The Fischer–Tropsch Synthesis in the Liquid Phase. Cat. Rev. 1980;21:225–274. doi: 10.1080/03602458008067534. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

