The Catalytic Degradation of Waste PU and the Preparation of Recycled Materials
- PMID: 39771435
- PMCID: PMC11679310
- DOI: 10.3390/polym16243581
The Catalytic Degradation of Waste PU and the Preparation of Recycled Materials
Abstract
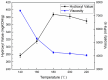
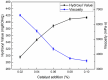
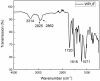
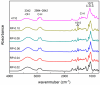
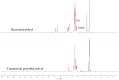
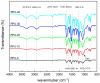
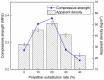
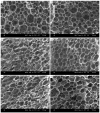
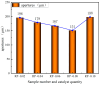
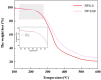
In this paper, we investigated the efficient metal-free phosphorus-nitrogen (PN) catalyst and used the PN catalyst to degrade waste PU with two-component binary mixed alcohols as the alcohol solvent. We examined the effects of reaction temperature, time, and other factors on the hydroxyl value and viscosity of the degradation products; focused on the changing rules of the hydroxyl value, viscosity, and molecular weight of polyols recovered from degradation products with different dosages of the metal-free PN catalyst; and determined the optimal experimental conditions of reaction temperature 180 °C, reaction time 3 h, and PN dosage 0.08%. The optimal experimental conditions were 180 °C, 3 h reaction time, and 0.08% PN dosage, the obtained polyol viscosity was 3716 mPa·s, the hydroxyl value was 409.2 mgKOH/g, and the number average molecular weight was 2616. The FTIR, 1H, NMR, and other tests showed that the waste urethanes were degraded into oligomers successfully, the recycled polyether polyols were obtained, and a series of recycled polyurethanes with different substitution ratios were then prepared. A series of recycled polyurethane materials with different substitution rates were then prepared and characterized by FTIR, SEM, compression strength, and thermal conductivity tests, which showed that the recycled polyurethane foams had good physical properties such as compression strength and apparent density, and the SEM test at a 20% substitution rate showed that the recycled polyol helped to improve the structure of the blisters.
Keywords: PN catalyst; alcoholysis; recycled materials; waste polyurethane.
Conflict of interest statement
The authors declare no competing financial or non-financial interests.
Figures







References
-
- Zhang G., Yin T., Nian G., Suo Z. Fatigue-resistant polyurethane elastomer composites. Extrem. Mech. Lett. 2021;48:101434. doi: 10.1016/j.eml.2021.101434. - DOI
-
- Watando H., Saya S., Fukaya T., Fujieda S., Yamamoto M. Improving chemical recycling rate by reclaiming polyurethane elastomer from polyurethane foam. Polym. Degrad. Stab. 2006;91:3354–3359. doi: 10.1016/j.polymdegradstab.2006.05.017. - DOI
-
- Sun M., Sheppard D.T., Brutman J.P., Alsbaiee A., Dichtel W.R. Green Catalysts for Reprocessing Thermoset Polyurethanes. Macromolecules. 2023;56:6978–6987. doi: 10.1021/acs.macromol.3c01116. - DOI
-
- Sadeghi G.M.M., Shamsi R., Sayaf M. From Aminolysis Product of PET Waste to Novel Biodegradable Polyurethanes. J. Polym. Environ. 2011;19:522–534. doi: 10.1007/s10924-011-0283-7. - DOI
LinkOut - more resources
Full Text Sources

