Manifestation of Donor-Acceptor Properties of N-Doped Polymer Carbon Dots During Hydrogen Bonds Formation in Different Solvents
- PMID: 39771437
- PMCID: PMC11679973
- DOI: 10.3390/polym16243585
Manifestation of Donor-Acceptor Properties of N-Doped Polymer Carbon Dots During Hydrogen Bonds Formation in Different Solvents
Abstract
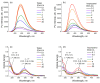
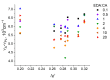
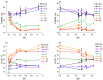
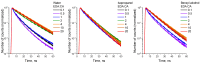
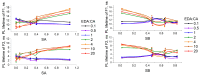
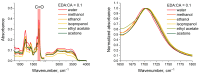
The effective use of polymer carbon dots (PCD) in various fields of science and technology requires a more detailed understanding of the mechanisms of their photoluminescence formation and change as a result of their interaction with the environment. In this study, PCD synthesized via a hydrothermal method from citric acid and ethylenediamine are studied in various solvents using FTIR spectroscopy, optical absorption spectroscopy, and photoluminescence spectroscopy. As a result of the analysis of the obtained dependencies of such PCD spectral characteristics as the photoluminescence FWHM, the photoluminescence quantum yield, the photoluminescence lifetime on the acidity and basicity of the solvent, a hypothesis was formulated on the formation mechanism of hydrogen bonds between the PCD surface groups and the molecules of the environment, and conclusions were made about the donor-acceptor nature of the synthesized PCD.
Keywords: absorption spectroscopy; acidity; basicity; donor–acceptor properties; hydrogen bonds; photoluminescence; photoluminescence lifetime; photoluminescence quantum yield; polymer carbon dots.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Ozyurt D., Kobaisi M.A., Hocking R.K., Fox B. Properties, Synthesis, and Applications of Carbon Dots: A Review. Carbon Trends. 2023;12:100276. doi: 10.1016/j.cartre.2023.100276. - DOI
-
- Wang B., Lu S. The Light of Carbon Dots: From Mechanism to Applications. Matter. 2022;5:110–149. doi: 10.1016/j.matt.2021.10.016. - DOI
-
- Liu H., Zhong X., Pan Q., Zhang Y., Deng W., Zou G., Hou H., Ji X. A Review of Carbon Dots in Synthesis Strategy. Coord. Chem. Rev. 2024;498:215468. doi: 10.1016/j.ccr.2023.215468. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

