Co-Amorphization, Dissolution, and Stability of Quench-Cooled Drug-Drug Coamorphous Supersaturating Delivery Systems with RT-Unstable Amorphous Components
- PMID: 39771470
- PMCID: PMC11677066
- DOI: 10.3390/pharmaceutics16121488
Co-Amorphization, Dissolution, and Stability of Quench-Cooled Drug-Drug Coamorphous Supersaturating Delivery Systems with RT-Unstable Amorphous Components
Abstract
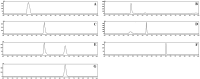
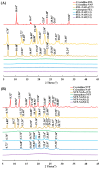
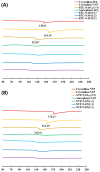
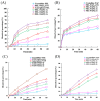
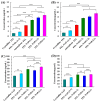
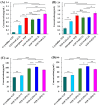
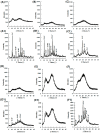
Background: Supersaturating drug delivery systems (SDDSs) have gained significant attention as a promising strategy to enhance the solubility and bioabsorption of Biopharmaceutics Classification System (BCS) II drugs. To overcome challenges associated with polymer-based amorphous SDDS (aSDDS), coamorphous (CAM) systems have emerged as a viable alternative. Among them, "drug-drug" CAM (ddCAM) systems show considerable potential for combination drug therapy. However, many drugs in their pure amorphous forms are unstable at room temperature (RT), complicating their formation and long-term stability profiles. Consequently, limited knowledge exists regarding the behavior of ddCAMs containing RT-unstable components formed via quench cooling. Methods: In this study, we used naproxen (NAP), a RT-unstable amorphous drug, in combination with felodipine (FEL) or nitrendipine (NTP), two RT-stable amorphous drugs, to create "FEL-NAP" and "NTP-NAP" ddCAM pairs via quench cooling. Our work used a series of methods to perform a detailed analysis on the co-amorphization, dissolution, solubility, and stability profiles of ddCAMs containing RT-unstable drugs, contributing to advancements in co-amorphization techniques for generating SDDS. Results: This study revealed that the co-amorphization and stability profiles of ddCAMs containing RT-unstable components produced via a quench-cooling method were closely related to drug-drug pairing types and ratios. Both quench-cooling and incorporation into coamorphous systems improved the dissolution, solubility, and physical stability of individual APIs. Conclusions: Our findings provide deeper insight into the co-amorphization, dissolution, and stability characteristics of specific drug-drug coamorphous systems FEL-NAP and NTP-NAP, offering valuable guidance for developing new ddCAM coamorphous formulations containing some RT-unstable drugs.
Keywords: coamorphization and stability; combination therapy; quench cooling; supersaturating drug delivery systems (SDDS); “drug–drug” coamorphous system (ddCAM).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
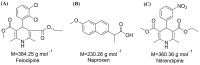



References
-
- Shah V.P., Amidon G.L. GL Amidon, H. Lennernas, VP Shah, and JR Crison. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability, Pharm Res 12, 413–420, 1995—Backstory of BC. AAPS J. 2014;16:894–898. doi: 10.1208/s12248-014-9620-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

