Arginine Hydrochloride Reduce Rectal Mucosal Irritation of Sodium Aescinate: Molecular Docking, Physical Properties, Anti-Hemorrhoidal Activity, Safety and Topical Gel Formulations Investigation
- PMID: 39771477
- PMCID: PMC11676831
- DOI: 10.3390/pharmaceutics16121498
Arginine Hydrochloride Reduce Rectal Mucosal Irritation of Sodium Aescinate: Molecular Docking, Physical Properties, Anti-Hemorrhoidal Activity, Safety and Topical Gel Formulations Investigation
Abstract
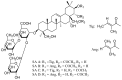
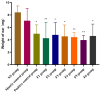
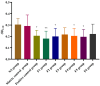
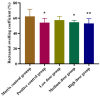
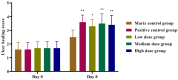
Background/Objectives: Sodium aescinate (SA) is commonly used topically due to its anti-inflammatory, anti-edematous, and anti-swelling properties. However, the clinical application of SA is limited by strong irritation, and cannot be used on the damaged skin and mucous membrane. This study aimed to investigate whether arginine hydrochloride (Arg·HCl) could reduce the rectal mucosal irritation of SA through the formation of a gel. Methods: Molecular docking was first used to explore potential interactions between SA and Arg·HCl. Gels for rectal administration were then formulated by combining SA with various ratios of Arg·HCl (from 1:0 to 1:10). In vitro tests, including pH, centrifuge stability, viscosity, and spreadability analysis, were conducted. The optimal gel formulation was determined based on rectal mucosal irritation tests and anti-inflammatory experiments. Additionally, the anti-hemorrhoidal characteristics and safety of the optimal gel in terms of acute toxicity and dermal sensitivity were evaluated. Results: The optimal SA to Arg·HCl ratio of 1:6 (F5-SA gel) was identified, significantly reducing rectal mucosal irritation while enhancing anti-inflammatory activity. The F5-SA gel demonstrated high efficacy against hemorrhoids, notably promoting anal ulcer healing. When administered rectally to rabbits at a dose of 132 mg·kg-1·d-1 (198 times the recommended therapeutic dose), no other obvious side effects were observed except a significant reduction in food intake on the day of administration. In addition, the gel did not induce dermal sensitivity. Conclusions: The F5-SA gel is a promising formulation that can reduce irritation and toxic side effects, and enhance the therapeutic effect to some extent, ultimately achieving a safer and more effective rectal delivery system for SA.
Keywords: arginine hydrochloride; gels; hemorrhoid; inflammation; rectal irritation; sodium aescinate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

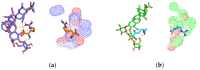
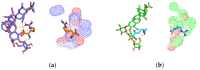



Similar articles
-
Modification of sodium aescinate into a safer, more stable and effective water-soluble drug by liposome-encapsulation: an in vitro and in vivo study.Drug Deliv. 2022 Dec;29(1):1132-1141. doi: 10.1080/10717544.2022.2058114. Drug Deliv. 2022. PMID: 35380084 Free PMC article.
-
Emulgel for improved topical delivery of Tretinoin: Formulation design and characterization.Ann Pharm Fr. 2022 Mar;80(2):157-168. doi: 10.1016/j.pharma.2021.05.004. Epub 2021 May 21. Ann Pharm Fr. 2022. PMID: 34029557
-
Development of an effective topical liposomal formulation for localized analgesia and anti-inflammatory actions in the Complete Freund's Adjuvant rodent model of acute inflammatory pain.Pain Physician. 2014 Nov-Dec;17(6):E719-35. Pain Physician. 2014. PMID: 25415787 Clinical Trial.
-
Final report of the safety assessment of Alcohol Denat., including SD Alcohol 3-A, SD Alcohol 30, SD Alcohol 39, SD Alcohol 39-B, SD Alcohol 39-C, SD Alcohol 40, SD Alcohol 40-B, and SD Alcohol 40-C, and the denaturants, Quassin, Brucine Sulfate/Brucine, and Denatonium Benzoate.Int J Toxicol. 2008;27 Suppl 1:1-43. doi: 10.1080/10915810802032388. Int J Toxicol. 2008. PMID: 18569160 Review.
-
Safety assessment of Salicylic Acid, Butyloctyl Salicylate, Calcium Salicylate, C12-15 Alkyl Salicylate, Capryloyl Salicylic Acid, Hexyldodecyl Salicylate, Isocetyl Salicylate, Isodecyl Salicylate, Magnesium Salicylate, MEA-Salicylate, Ethylhexyl Salicylate, Potassium Salicylate, Methyl Salicylate, Myristyl Salicylate, Sodium Salicylate, TEA-Salicylate, and Tridecyl Salicylate.Int J Toxicol. 2003;22 Suppl 3:1-108. Int J Toxicol. 2003. PMID: 14617432 Review.
References
-
- Bombardelli E., Morazzoni P., Griffini A. Aesculus hippocastanum L. Fitoterapia. 1996;67:483–511.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

