Concomitant Inhibition and Collaring of Dual-Species Biofilms Formed by Candida auris and Staphylococcus aureus by Triazole Based Small Molecule Inhibitors
- PMID: 39771549
- PMCID: PMC11677466
- DOI: 10.3390/pharmaceutics16121570
Concomitant Inhibition and Collaring of Dual-Species Biofilms Formed by Candida auris and Staphylococcus aureus by Triazole Based Small Molecule Inhibitors
Abstract
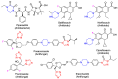
Background/objectives: Biofilm-associated infections, particularly those involving Candida auris and Staphylococcus aureus, pose significant challenges in clinical settings due to their resilience and resistance to conventional treatments. This study aimed to synthesize novel triazole derivatives containing a piperazine ring via click chemistry and evaluate their efficacy in disrupting biofilms formed by these pathogens.
Methods: Triazole derivatives were synthesized using click chemistry techniques. The antimicrobial activity of the compounds was tested against planktonic cells of C. auris and S. aureus in single and dual-species culture conditions. Biofilm disruption efficacy was assessed, alongside the evaluation of physicochemical properties, oral bioavailability potential, and toxicity profiles.
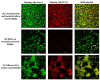
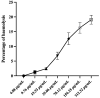
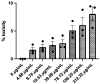
Results: The compound T3 demonstrated potent antimicrobial activity against planktonic cells of C. auris and S. aureus in both single and dual-species cultures. T3 exhibited significant efficacy in reducing microbial viability within biofilms formed by these pathogens. Physicochemical analyses revealed favorable solubility and permeability profiles, supporting its potential for oral bioavailability. Toxicity assessments showed a non-toxic profile, highlighting a promising safety margin for further development.
Conclusions: This study underscores the anti-biofilm properties of novel triazole-piperazine derivatives, particularly T3, against single and dual-species biofilms of C. auris and S. aureus. These findings position T3 as a promising candidate for developing therapies targeting polymicrobial infections and provide a foundation for future research into alternative strategies for combating biofilm-associated infections.
Keywords: biofilms; click chemistry; drug resistance; physicochemical properties; triazole.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures









Similar articles
-
The antimicrobial and antibiofilm effects of gentamicin, imipenem, and fucoidan combinations against dual-species biofilms of Staphylococcus aureus and Acinetobacter baumannii isolated from diabetic foot ulcers.Ann Clin Microbiol Antimicrob. 2024 Nov 15;23(1):101. doi: 10.1186/s12941-024-00760-w. Ann Clin Microbiol Antimicrob. 2024. PMID: 39548455 Free PMC article.
-
Antibiofilm efficacy of the gold compound auranofin on dual species biofilms of Staphylococcus aureus and Candida sp.J Appl Microbiol. 2020 Jan;128(1):88-101. doi: 10.1111/jam.14443. Epub 2019 Nov 19. J Appl Microbiol. 2020. PMID: 31509623
-
Efficacy of biofilm disrupters against Candida auris and other Candida species in monomicrobial and polymicrobial biofilms.Mycoses. 2024 Jan;67(1):e13684. doi: 10.1111/myc.13684. Mycoses. 2024. PMID: 38214428
-
Inhibition of apoptosis and biofilm formation in Candida auris by click-synthesized triazole-bridged quinoline derivatives.RSC Adv. 2024 Jul 4;14(29):21190-21202. doi: 10.1039/d4ra03728f. eCollection 2024 Jun 27. RSC Adv. 2024. PMID: 38966810 Free PMC article.
-
Nanoparticles in the battle against Candida auris biofilms: current advances and future prospects.Drug Deliv Transl Res. 2025 May;15(5):1496-1512. doi: 10.1007/s13346-024-01749-w. Epub 2024 Nov 26. Drug Deliv Transl Res. 2025. PMID: 39589626 Free PMC article. Review.
Cited by
-
Rosemary Extract: Phytochemical Composition and Potential for Eliminating Polymicrobial Biofilm of Candida albicans and Multidrug-Resistant Bacteria.BioTech (Basel). 2025 Aug 13;14(3):61. doi: 10.3390/biotech14030061. BioTech (Basel). 2025. PMID: 40843784 Free PMC article.
-
Chitosan-Based Hydrogels Containing Nystatin and Propolis as a Novel Tool for Candida auris Skin Decolonization.Gels. 2025 Jun 26;11(7):498. doi: 10.3390/gels11070498. Gels. 2025. PMID: 40710659 Free PMC article.
References
-
- Welsh R.M., Bentz M.L., Shams A., Houston H., Lyons A., Rose L.J., Litvintseva A.P. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J. Clin. Microbiol. 2017;55:2996–3005. doi: 10.1128/JCM.00921-17. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

