Development and Evaluation of Five-in-One Vaccine Microneedle Array Patch for Diphtheria, Tetanus, Pertussis, Hepatitis B, and Haemophilus influenzae Type b: Immunological Efficacy and Long-Term Stability
- PMID: 39771609
- PMCID: PMC11677855
- DOI: 10.3390/pharmaceutics16121631
Development and Evaluation of Five-in-One Vaccine Microneedle Array Patch for Diphtheria, Tetanus, Pertussis, Hepatitis B, and Haemophilus influenzae Type b: Immunological Efficacy and Long-Term Stability
Abstract
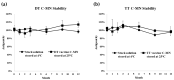
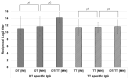
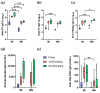
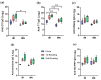
Background and objectives: The development of a five-in-one vaccine microneedle patch (five-in-one MN patch) aims to address challenges in administering vaccines against Diphtheria (DT), Tetanus (TT), Pertussis (wP), Hepatitis B (HBsAg), and Haemophilus influenzae type b (Hib). Combining multiple vaccines into a single patch offers a novel solution to improve vaccine accessibility, stability, and delivery efficiency, particularly in resource-limited settings. Methods: The five-in-one MN patch consists of four distinct microneedle arrays: DT and TT vaccines are coated together on one array, while wP, HepB, and Hib vaccines are coated separately on individual arrays. The patch was tested for long-term stability (12 months at 25 °C) and evaluated for immunogenicity in mice and minipigs. Antibody titers were measured using ELISA to compare immune responses between microneedle-based delivery and traditional intramuscular (IM) injection. Results: The five-in-one MN patch demonstrated stable antigenicity for up to 12 months at room temperature. In animal studies, the patch induced antibody titers comparable to traditional IM injections for all vaccines. Notably, immunogenic responses to Pertussis and Haemophilus influenzae type b vaccines via microneedles were reported for the first time. The patch facilitated the simultaneous yet independent delivery of vaccines, preserving their immunogenicity without interference. Conclusions: The five-in-one MN patch represents a significant advancement in vaccine delivery by enabling stable, minimally invasive, and efficient immunization. Its innovative design addresses the critical limitations of combination vaccines and has the potential to enhance vaccine accessibility in low- and middle-income countries. Future studies will focus on optimizing patch application techniques and evaluating broader clinical applicability.
Keywords: combination vaccine; immunological efficacy; microneedle array; microneedle patch; thermal stability.
Conflict of interest statement
Author I.-J.C., D.K., J.S.K., J.K., J.-E.C., A.K. and S.-K.B. were employed by QuadMedicine Inc. and author T.-H.K. was employed by LG Chemical Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Hib antibody responses in infants following diphtheria, tetanus, acellular pertussis, and conjugated Haemophilus influenzae type b (Hib) combination vaccines with decreasing amounts of tetanus toxoid.Vaccine. 2017 Dec 4;35(48 Pt B):6707-6711. doi: 10.1016/j.vaccine.2017.10.016. Epub 2017 Oct 18. Vaccine. 2017. PMID: 29054729 Clinical Trial.
-
Use of lidocaine-prilocaine patch to decrease intramuscular injection pain does not adversely affect the antibody response to diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b conjugate and hepatitis B vaccines in infants from birth to six months of age.Pediatr Infect Dis J. 2002 May;21(5):399-405. doi: 10.1097/00006454-200205000-00010. Pediatr Infect Dis J. 2002. PMID: 12150176 Clinical Trial.
-
Co-administration of a novel Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine does not interfere with the immune response to antigens contained in infant vaccines routinely used in the United States.Hum Vaccin. 2011 Feb;7(2):258-64. doi: 10.4161/hv.7.2.14170. Epub 2011 Feb 1. Hum Vaccin. 2011. PMID: 21307655 Clinical Trial.
-
Integration of hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B virus, inactivated poliomyelitis and Haemophilus influenzae type b conjugate vaccine within existing national recommendations following a birth dose of monovalent hepatitis B virus vaccine: results of a systematic review in the Asia Pacific region.Expert Rev Vaccines. 2019 Sep;18(9):921-933. doi: 10.1080/14760584.2019.1646643. Epub 2019 Aug 1. Expert Rev Vaccines. 2019. PMID: 31328999
-
Spotlight on DTPa-HBV-IPV/Hib Vaccine (Infanrix hexa).BioDrugs. 2010 Oct 1;24(5):299-302. doi: 10.2165/11206690-000000000-00000. BioDrugs. 2010. PMID: 20795752 Review.
Cited by
-
Beyond the Needle: Innovative Microneedle-Based Transdermal Vaccination.Medicines (Basel). 2025 Feb 7;12(1):4. doi: 10.3390/medicines12010004. Medicines (Basel). 2025. PMID: 39982324 Free PMC article. Review.
References
-
- Giersing B., Shah N., Kristensen D., Amorij J.-P., Kahn A.-L., Gandrup-Marino K., Jarrahian C., Zehrung D., Menozzi-Arnaud M. Strategies for vaccine-product innovation: Creating an enabling environment for product development to uptake in low-and middle-income countries. Vaccine. 2021;39:7208–7219. doi: 10.1016/j.vaccine.2021.07.091. - DOI - PMC - PubMed
-
- Susarla S.K., Gupta M., Mantan M., Dhongade R., Bhave S., Das R.K., Ray R.K., Babu T.R., Ravi M., Krishnamurthy B. Immunogenicity and safety of a liquid pentavalent (DTwP-Hb-Hib) combination vaccine manufactured by human biologicals institute in 6–8 weeks old healthy infants: A phase III, randomized, single blind, non-inferiority study. Vaccine. 2019;37:5452–5459. doi: 10.1016/j.vaccine.2019.06.067. - DOI - PubMed
-
- Gupta M., Kumar R., Deb A.K., Bhattacharya S.K., Bose A., John J., Balraj V., Ganguly N., Kant L., Kapoor A.N., et al. Multi-center surveillance for pneumonia & meningitis among children (<2 yr) for Hib vaccine probe trial preparation in India. Indian J. Med. Res. 2010;131:649–658. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

