Detection and Quantification of DNA by Fluorophore-Induced Plasmonic Current: A Novel Sensing Approach
- PMID: 39771720
- PMCID: PMC11679120
- DOI: 10.3390/s24247985
Detection and Quantification of DNA by Fluorophore-Induced Plasmonic Current: A Novel Sensing Approach
Abstract
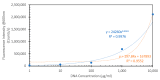
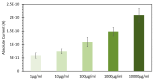
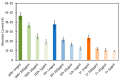
We report on the detection and quantification of aqueous DNA by a fluorophore-induced plasmonic current (FIPC) sensing method. FIPC is a mechanism described by our group in the literature where a fluorophore in close proximity to a plasmonically active metal nanoparticle film (MNF) is able to couple with it, when in an excited state. This coupling produces enhanced fluorescent intensity from the fluorophore-MNF complex, and if conditions are met, a current is generated in the film that is intrinsically linked to the properties of the fluorophore in the complex. The magnitude of this induced current is related to the spectral properties of the film, the overlap between these film properties and those of the fluorophore, the spacing between the nanoparticles in the film, the excitation wavelength, and the polarization of the excitation source. Recent literature has shown that the FIPC system is ideal for aqueous ion sensing using turn-on fluorescent probes, and in this paper, we subsequently examine if it is possible to detect aqueous DNA also via a turn-on fluorescent probe, as well as other commercially available DNA detection strategies. We report the effects of DNA concentration, probe concentration, and probe characteristics on the development of an FIPC assay for the detection of non-specific DNA in aqueous solutions.
Keywords: DNA sensing; fluorophore induced plasmonic current; metal-enhanced fluorescence; plasmonic current; plasmonic electricity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











Similar articles
-
Direct Detection and Quantification of Aqueous Proteins via a Fluorescent Probe Through the Use of Fluorophore-Induced Plasmonic Current.Biosensors (Basel). 2025 Feb 27;15(3):150. doi: 10.3390/bios15030150. Biosensors (Basel). 2025. PMID: 40136947 Free PMC article.
-
Fluorophore-Induced Plasmonic Current Generation from Aluminum Nanoparticle Films.J Phys Chem C Nanomater Interfaces. 2023 Jan 19;127(2):1126-1134. doi: 10.1021/acs.jpcc.2c07588. Epub 2023 Jan 4. J Phys Chem C Nanomater Interfaces. 2023. PMID: 38106338 Free PMC article.
-
Fluorophore-Induced Plasmonic Current Generation from Copper Nanoparticle Films.ACS Omega. 2024 May 24;9(23):25181-25188. doi: 10.1021/acsomega.4c02751. eCollection 2024 Jun 11. ACS Omega. 2024. PMID: 38882126 Free PMC article.
-
Localized surface plasmon resonance: a unique property of plasmonic nanoparticles for nucleic acid detection.Nanoscale. 2013 Dec 21;5(24):12043-71. doi: 10.1039/c3nr02257a. Nanoscale. 2013. PMID: 24166199 Review.
-
Plasmonic Nanomaterial-Based Optical Biosensing Platforms for Virus Detection.Sensors (Basel). 2017 Oct 13;17(10):2332. doi: 10.3390/s17102332. Sensors (Basel). 2017. PMID: 29027923 Free PMC article. Review.
References
-
- Lakowicz J.R. Principles of Fluorescence Spectroscopy. Springer; New York, NY, USA: 2006.
-
- Geddes C.D., Aslan K. Metal-Enhanced Fluorescence: Progress Towards a Unified Plasmon-Fluorophore Description. John Wiley and Sons; Hoboken, NJ, USA: 2010.
-
- Pelton M., Bryant G.W. Introduction to Metal-Nanoparticle Plasmonics. John Wiley & Sons; Hoboken, NJ, USA: 2013.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

