Optically Controlled Drug Delivery Through Microscale Brain-Machine Interfaces Using Integrated Upconverting Nanoparticles
- PMID: 39771721
- PMCID: PMC11680031
- DOI: 10.3390/s24247987
Optically Controlled Drug Delivery Through Microscale Brain-Machine Interfaces Using Integrated Upconverting Nanoparticles
Abstract
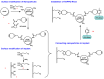
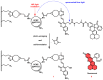
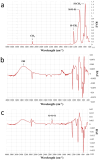
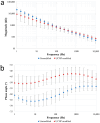
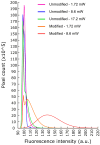
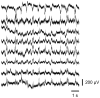
The aim of this work is to incorporate lanthanide-cored upconversion nanoparticles (UCNP) into the surface of microengineered biomedical implants to create a spatially controlled and optically releasable model drug delivery device in an integrated fashion. Our approach enables silicone-based microelectrocorticography (ECoG) implants holding platinum/iridium recording sites to serve as a stable host of UCNPs. Nanoparticles excitable in the near-infrared (lower energy) regime and emitting visible (higher energy) light are utilized in a study. With the upconverted higher energy photons, we demonstrate the induction of photochemical (cleaving) reactions that enable the local release of specific dyes as a model system near the implant. The modified ECoG electrodes can be implanted in brain tissue to act as an uncaging system that releases small amounts of substance while simultaneously measuring the evoked neural response upon light activation. In this paper, several technological challenges like the surface modification of UCNPs, the immobilization of particles on the implantable platform, and measuring the stability of integrated UCNPs in in vitro and in vivo conditions are addressed in detail. Besides the chemical, mechanical, and optical characterization of the ready-to-use devices, the effect of nanoparticles on the original electrophysiological function is also evaluated. The results confirm that silicone-based brain-machine interfaces can be efficiently complemented with UCNPs to facilitate local model drug release.
Keywords: biomedical implant; brain–machine interfaces; drug delivery; electrocorticography; upconverting nanoparticles.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
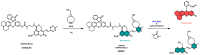



Similar articles
-
Engineered lanthanide-doped upconversion nanoparticles for biosensing and bioimaging application.Mikrochim Acta. 2022 Feb 17;189(3):109. doi: 10.1007/s00604-022-05180-1. Mikrochim Acta. 2022. PMID: 35175435 Review.
-
Near-infrared photochemistry at interfaces based on upconverting nanoparticles.Phys Chem Chem Phys. 2017 Sep 13;19(35):23585-23596. doi: 10.1039/c7cp01838j. Phys Chem Chem Phys. 2017. PMID: 28480940
-
Emerging NIR light-responsive delivery systems based on lanthanide-doped upconverting nanoparticles.Arch Pharm Res. 2020 Jan;43(1):134-152. doi: 10.1007/s12272-020-01208-3. Epub 2020 Jan 24. Arch Pharm Res. 2020. PMID: 31981073 Review.
-
Self-assembled photoadditives in polyester films allow stop and go chemical release.Acta Biomater. 2017 May;54:186-200. doi: 10.1016/j.actbio.2017.03.021. Epub 2017 Mar 16. Acta Biomater. 2017. PMID: 28315815
-
Modularly Assembled Upconversion Nanoparticles for Orthogonally Controlled Cell Imaging and Drug Delivery.ACS Appl Mater Interfaces. 2020 Mar 18;12(11):12549-12556. doi: 10.1021/acsami.0c00672. Epub 2020 Mar 6. ACS Appl Mater Interfaces. 2020. PMID: 32100992
References
-
- Patra J.K., Das G., Fraceto L.F., Campos E.V.R., del Pilar Rodriguez-Torres M., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. - DOI - PMC - PubMed
-
- Luong H.V.T., Diep M.T., Nguyen N.Y., Pham D.T., Cao L.N.H., Ha T.M.P. Alginate–functionalized Fe3O4 nanoparticles as a drug delivery system for targeted controlled release. J. Drug Deliv. Sci. Technol. 2024;93:105465. doi: 10.1016/j.jddst.2024.105465. - DOI
MeSH terms
Grants and funding
- TKP2021-EGA-42/National Research, Development and Innovation Office
- TKP2021-EGA-04/National Research, Development and Innovation Office
- VKE-2018-00032/National Research, Development and Innovation Office
- KFI-2018-00097/National Research, Development and Innovation Office
- 2020-2.1.1-ED-2022-00208/National Research, Development and Innovation Office
LinkOut - more resources
Full Text Sources

