Image Synthesis in Nuclear Medicine Imaging with Deep Learning: A Review
- PMID: 39771804
- PMCID: PMC11679239
- DOI: 10.3390/s24248068
Image Synthesis in Nuclear Medicine Imaging with Deep Learning: A Review
Abstract
Nuclear medicine imaging (NMI) is essential for the diagnosis and sensing of various diseases; however, challenges persist regarding image quality and accessibility during NMI-based treatment. This paper reviews the use of deep learning methods for generating synthetic nuclear medicine images, aimed at improving the interpretability and utility of nuclear medicine protocols. We discuss advanced image generation algorithms designed to recover details from low-dose scans, uncover information hidden by specific radiopharmaceutical properties, and enhance the sensing of physiological processes. By analyzing 30 of the newest publications in this field, we explain how deep learning models produce synthetic nuclear medicine images that closely resemble their real counterparts, significantly enhancing diagnostic accuracy when images are acquired at lower doses than the clinical policies' standard. The implementation of deep learning models facilitates the combination of NMI with various imaging modalities, thereby broadening the clinical applications of nuclear medicine. In summary, our review underscores the significant potential of deep learning in NMI, indicating that synthetic image generation may be essential for addressing the existing limitations of NMI and improving patient outcomes.
Keywords: nuclear medicine imaging; synthesizing; transforming.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


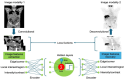
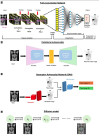



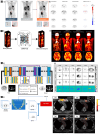
Similar articles
-
Deep learning in nuclear medicine: from imaging to therapy.Ann Nucl Med. 2025 May;39(5):424-440. doi: 10.1007/s12149-025-02031-w. Epub 2025 Mar 13. Ann Nucl Med. 2025. PMID: 40080372 Review.
-
Pathology Image Analysis Using Segmentation Deep Learning Algorithms.Am J Pathol. 2019 Sep;189(9):1686-1698. doi: 10.1016/j.ajpath.2019.05.007. Epub 2019 Jun 11. Am J Pathol. 2019. PMID: 31199919 Free PMC article. Review.
-
Artificial Intelligence in Nuclear Cardiology.J Nucl Med. 2019 Aug;60(8):1042-1043. doi: 10.2967/jnumed.118.222356. Epub 2019 Mar 29. J Nucl Med. 2019. PMID: 30926652 No abstract available.
-
Deep learning techniques in PET/CT imaging: A comprehensive review from sinogram to image space.Comput Methods Programs Biomed. 2024 Jan;243:107880. doi: 10.1016/j.cmpb.2023.107880. Epub 2023 Oct 21. Comput Methods Programs Biomed. 2024. PMID: 37924769 Review.
-
The Evolution of Artificial Intelligence in Nuclear Medicine.Semin Nucl Med. 2025 May;55(3):313-327. doi: 10.1053/j.semnuclmed.2025.01.006. Epub 2025 Feb 10. Semin Nucl Med. 2025. PMID: 39934005 Review.
Cited by
-
Nanoradiopharmaceuticals: Design Principles, Radiolabeling Strategies, and Biomedicine Applications.Pharmaceutics. 2025 Jul 14;17(7):912. doi: 10.3390/pharmaceutics17070912. Pharmaceutics. 2025. PMID: 40733120 Free PMC article. Review.
References
-
- Le D. An Overview of the Regulations of Radiopharmaceuticals. In: Wong F.C.L., editor. Locoregional Radionuclide Cancer Therapy: Clinical and Scientific Aspects. Springer International Publishing; Cham, Switzerland: 2021. pp. 225–247.
-
- Townsend D.W., Carney J.P.J., Yap J.T., Hall N.C. PET/CT Today and Tomorrow. J. Nucl. Med. 2004;45:4S–14S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NRF-2022R1I1A3068823, NRF-2022M3A9E4017151/National Research Foundation (NRF)
- IITP-2023-RS-2023-00256629/Institute of Information & communications Technology Planning & Evaluation (IITP) under the Artificial Intelligence Convergence Innovation Human Resources Development
- HCRI23029/Chonnam National University Hwasun Hospital Institute for Biomedical Science
LinkOut - more resources
Full Text Sources

