Light-Emitting Diode Array with Optical Linear Detector Enables High-Throughput Differential Single-Cell Dielectrophoretic Analysis
- PMID: 39771806
- PMCID: PMC11679556
- DOI: 10.3390/s24248071
Light-Emitting Diode Array with Optical Linear Detector Enables High-Throughput Differential Single-Cell Dielectrophoretic Analysis
Abstract
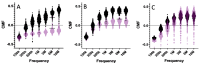
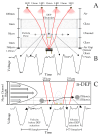
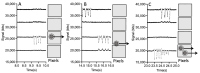
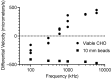
This paper presents a lens-free imaging approach utilizing an array of light sources, capable of measuring the dielectric properties of many particles simultaneously. This method employs coplanar electrodes to induce velocity changes in flowing particles through dielectrophoretic forces, allowing the inference of individual particle properties from differential velocity changes. Both positive and negative forces are detectable. The light source utilized in this system is composed of LEDs with a wavelength of 470 nm, while detection is performed using a 256-element optical array detector. Measurements with 10 μm polystyrene beads demonstrate this method can resolve changes equivalent to a Clausius-Mossotti factor of 0.18. Simulations in this work, using values from the literature, predict that Clausius-Mossotti factor differences of 0.18 are sufficient to differentiate viable from nonviable cells and cancerous from multidrug-resistant cancerous cells. We demonstrate that for Chinese hamster ovary (CHO) cells, the method can collect a dielectric response spectrum for a large number of cells in several minutes. We demonstrate that for CHO cells, Clausius-Mossotti factor differences of 0.18 can be discriminated. Due to its simple detection apparatus and the utilization of high-throughput, wide, clog-resistant channels, this method holds promise for a wide range of applications.
Keywords: dielectric spectrum; dielectrophoresis; differential detection; in-flow analysis; lens-free; single-cell sensor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Parallel single-cell optical transit dielectrophoresis cytometer.Electrophoresis. 2020 May;41(9):720-728. doi: 10.1002/elps.201900393. Epub 2020 Feb 19. Electrophoresis. 2020. PMID: 32043614
-
Combined Dielectric-Optical Characterization of Single Cells Using Dielectrophoresis-Imaging Flow Cytometry.Biosensors (Basel). 2024 Nov 27;14(12):577. doi: 10.3390/bios14120577. Biosensors (Basel). 2024. PMID: 39727842 Free PMC article.
-
Light-induced dielectrophoretic manipulation of DNA.Biophys J. 2007 Aug 1;93(3):1032-8. doi: 10.1529/biophysj.106.101188. Epub 2007 May 4. Biophys J. 2007. PMID: 17483160 Free PMC article.
-
Electric field-induced effects on neuronal cell biology accompanying dielectrophoretic trapping.Adv Anat Embryol Cell Biol. 2003;173:III-IX, 1-77. doi: 10.1007/978-3-642-55469-8. Adv Anat Embryol Cell Biol. 2003. PMID: 12901336 Review.
-
Signal-Based Methods in Dielectrophoresis for Cell and Particle Separation.Biosensors (Basel). 2022 Jul 11;12(7):510. doi: 10.3390/bios12070510. Biosensors (Basel). 2022. PMID: 35884313 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

