Safety, Immunogenicity and Protective Activity of a Modified Trivalent Live Attenuated Influenza Vaccine for Combined Protection Against Seasonal Influenza and COVID-19 in Golden Syrian Hamsters
- PMID: 39771962
- PMCID: PMC11679497
- DOI: 10.3390/vaccines12121300
Safety, Immunogenicity and Protective Activity of a Modified Trivalent Live Attenuated Influenza Vaccine for Combined Protection Against Seasonal Influenza and COVID-19 in Golden Syrian Hamsters
Abstract
Background/objectives: Influenza viruses and SARS-CoV-2 are currently cocirculating with similar seasonality, and both pathogens are characterized by a high mutational rate which results in reduced vaccine effectiveness and thus requires regular updating of vaccine compositions. Vaccine formulations combining seasonal influenza and SARS-CoV-2 strains can be considered promising and cost-effective tools for protection against both infections.
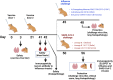
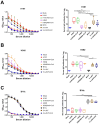
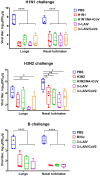
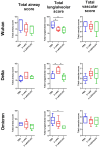
Methods: We used a licensed seasonal trivalent live attenuated influenza vaccine (3×LAIV) as a basis for the development of a modified 3×LAIV/CoV-2 vaccine, where H1N1 and H3N2 LAIV strains encoded an immunogenic cassette enriched with conserved T-cell epitopes of SARS-CoV-2, whereas a B/Victoria lineage LAIV strain was unmodified. The trivalent LAIV/CoV-2 composition was compared to the classical 3×LAIV in the golden Syrian hamster model. Animals were intranasally immunized with the mixtures of the vaccine viruses, twice, with a 3-week interval. Immunogenicity was assessed on day 42 of the study, and the protective effect was established by infecting vaccinated hamsters with either influenza H1N1, H3N2 or B viruses or with SARS-CoV-2 strains of the Wuhan, Delta and Omicron lineages.
Results: Both the classical 3×LAIV and 3×LAIV/CoV-2 vaccine compositions induced similar levels of serum antibodies specific to all three influenza strains, which resulted in comparable levels of protection against challenge from either influenza strain. Protection against SARS-CoV-2 challenge was more pronounced in the 3×LAIV/CoV-2-immunized hamsters compared to the classical 3×LAIV group. These data were accompanied by the higher magnitude of virus-specific cellular responses detected by ELISPOT in the modified trivalent LAIV group.
Conclusions: The modified trivalent live attenuated influenza vaccine encoding the T-cell epitopes of SARS-CoV-2 can be considered a promising tool for combined protection against seasonal influenza and COVID-19.
Keywords: LAIV; SARS-CoV-2; Syrian hamster; bivalent vaccine; influenza virus; preclinical research.
Conflict of interest statement
K.K. and V.M. (Valery Makarov) are employees of the Research-and-Manufacturing Company “Home of Pharmacy”. E.S., V.M. (Victoria Matyushenko), L.R. and I.I.-S. are inventors of planned patent on this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- WHO Influenza (Seasonal) [(accessed on 19 September 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
-
- Caini S., Meijer A., Nunes M.C., Henaff L., Zounon M., Boudewijns B., Del Riccio M., Paget J. Probable Extinction of Influenza B/Yamagata and Its Public Health Implications: A Systematic Literature Review and Assessment of Global Surveillance Databases. Lancet Microbe. 2024;5:100851. doi: 10.1016/S2666-5247(24)00066-1. - DOI - PubMed
-
- Moro P.L., Zhang B., Ennulat C., Harris M., McVey R., Woody G., Marquez P., McNeil M.M., Su J.R. Safety of Co-Administration of mRNA COVID-19 and Seasonal Inactivated Influenza Vaccines in the Vaccine Adverse Event Reporting System (VAERS) during July 1, 2021–June 30, 2022. Vaccine. 2023;41:1859. doi: 10.1016/j.vaccine.2022.12.069. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

