Monitoring the Risk of Type-2 Circulating Vaccine-Derived Poliovirus Emergence During Roll-Out of Type-2 Novel Oral Polio Vaccine
- PMID: 39771970
- PMCID: PMC11680014
- DOI: 10.3390/vaccines12121308
Monitoring the Risk of Type-2 Circulating Vaccine-Derived Poliovirus Emergence During Roll-Out of Type-2 Novel Oral Polio Vaccine
Abstract
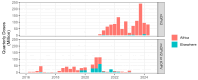
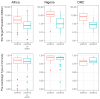
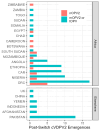
Background/Objectives: Although wild poliovirus type 2 has been eradicated, the prolonged transmission of the live- attenuated virus contained in the type-2 oral polio vaccine (OPV2) in under-immunized populations has led to the emergence of circulating vaccine-derived poliovirus type 2 (cVDPV2). The novel OPV2 (nOPV2) was designed to be more genetically stable and reduce the chance of cVDPV2 emergence while retaining comparable immunogenicity to the Sabin monovalent OPV2 (mOPV2). This study aimed to estimate the relative reduction in the emergence risk due to the use of nOPV2 instead of mOPV2. Methods: Data on OPV2 vaccination campaigns from May 2016 to 1 August 2024 were analyzed to estimate type-2 OPV-induced immunity in children under 5 years of age. Poliovirus surveillance data were used to estimate seeding dates and classify cVDPV2 emergences as mOPV2- or nOPV2-derived. The expected number of emergences if mOPV2 was used instead of nOPV2 was estimated, accounting for the timing and volume of nOPV2 doses, the known risk factors for emergence from mOPV2, and censoring due to the incomplete observation period for more recent nOPV2 doses. Results: As of 1 August 2024, over 98% of the approximately 1.19 billion nOPV2 doses administered globally were in Africa. We estimate that approximately 76 (95% confidence interval 69-85) index isolates of cVDPV2 emergences would be expected to be detected by 1 August 2024 if mOPV2 had been used instead of nOPV2 in Africa. The 18 observed nOPV2-derived emergences represent a 76% (74-79%) lower risk of emergence by nOPV2 than mOPV2 in Africa. The crude global analysis produced similar results. Key limitations include the incomplete understanding of the drivers of heterogeneity in emergence risk across geographies and variance in the per-dose risk of emergence may be incompletely captured using known risk factors. Conclusions: These results are consistent with the accumulating clinical and field evidence showing the enhanced genetic stability of nOPV2 relative to mOPV2, and this approach has been implemented in near-real time to contextualize new findings during the roll-out of this new vaccine. While nOPV2 has resulted in new emergences of cVDPV2, the number of cVDPV2 emergences is estimated to be approximately four-fold lower than if mOPV2 had been used instead.
Keywords: circulating vaccine-derived poliovirus; emergence risk; novel oral polio vaccine; polio.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Effectiveness of poliovirus vaccines against circulating vaccine-derived type 2 poliomyelitis in Nigeria between 2017 and 2022: a case-control study.Lancet Infect Dis. 2024 Apr;24(4):427-436. doi: 10.1016/S1473-3099(23)00688-6. Epub 2024 Jan 18. Lancet Infect Dis. 2024. PMID: 38246190
-
Update on Vaccine-Derived Poliovirus Outbreaks - Worldwide, July 2019-February 2020.MMWR Morb Mortal Wkly Rep. 2020 Apr 24;69(16):489-495. doi: 10.15585/mmwr.mm6916a1. MMWR Morb Mortal Wkly Rep. 2020. PMID: 32324719 Free PMC article.
-
Vaccine-derived poliovirus serotype 2 outbreaks and response in the Democratic Republic of the Congo, 2017-2021.Vaccine. 2023 Apr 6;41 Suppl 1(Suppl 1):A35-A47. doi: 10.1016/j.vaccine.2023.02.042. Epub 2023 Mar 11. Vaccine. 2023. PMID: 36907733 Free PMC article.
-
Looking back at prospective modeling of outbreak response strategies for managing global type 2 oral poliovirus vaccine (OPV2) cessation.Front Public Health. 2023 Mar 24;11:1098419. doi: 10.3389/fpubh.2023.1098419. eCollection 2023. Front Public Health. 2023. PMID: 37033033 Free PMC article. Review.
-
Novel Oral Polio Vaccine Type 2 Use for Polio Outbreak Response: A Global Effort for a Global Health Emergency.Pathogens. 2024 Mar 23;13(4):273. doi: 10.3390/pathogens13040273. Pathogens. 2024. PMID: 38668228 Free PMC article. Review.
Cited by
-
Geographic disparities impacting oral vaccine performance: Observations and future directions.Clin Exp Immunol. 2025 Jan 21;219(1):uxae124. doi: 10.1093/cei/uxae124. Clin Exp Immunol. 2025. PMID: 39774633 Review.
-
Impact of novel Oral Poliovirus Type 2 vaccination campaigns: A seroprevalence survey in Nigeria, 2022.Vaccine. 2025 Apr 30;54:126978. doi: 10.1016/j.vaccine.2025.126978. Epub 2025 Mar 19. Vaccine. 2025. PMID: 40112661 Free PMC article. No abstract available.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

