Differential Neutralization Profiles of 17DD Vaccinated Population to 17D-204 and 17DD Vaccine Strains
- PMID: 39771973
- PMCID: PMC11679407
- DOI: 10.3390/vaccines12121311
Differential Neutralization Profiles of 17DD Vaccinated Population to 17D-204 and 17DD Vaccine Strains
Abstract
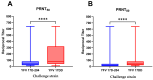
Background/Objectives: Yellow fever virus (YFV) (Flaviviridae, Orthoflavivirus) is the etiologic agent of yellow fever (YF), a vector-borne disease with significant morbidity and mortality across the tropics and neotropics, despite having a highly efficacious and safe vaccine (17D). Vaccination provides lifelong protection from YF disease mediated by humoral immunity. There are several versions of the original 17D vaccine: 17D-204 (marketed in the USA as YF-VAX, in France as Stamaril, and in China as Tiantan-V), 17D-213 (Russian Federation), and 17DD (by FIOCRUZ in Brazil). Vaccines produced in the US, France, Senegal, China, and Russia represent 17D-204-derived strains, whereas the Brazilian 17DD has a unique passage/attenuation history from 17D-204-derived strains. Their functional differences in the neutralization profiles are not known. Methods: The Plaque Reduction Neutralization Test (PRNT) was used to determine the neutralization profiles of sera from 209 patients that were previously vaccinated with the 17DD strain against both 17D-204 and 17DD. Results: Sera exhibited significantly more efficient neutralization of 17DD (mean reciprocal PRNT50 183, PRNT80 86, median reciprocal PRNT50 80, and PRNT80 40) compared to 17D-204 (mean reciprocal PRNT50 91, PRNT80 33, median reciprocal PRNT50 40, and PRNT80 10). Conclusions: Our data indicate antigenic differences between 17D and 17DD vaccines.
Keywords: YFV; immune response; neutralizing antibodies; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- ICTV . Virus Taxonomy: 2023 Release (MSL #39) ICTV; Jena, Germany: 2023. [(accessed on 24 September 2024)]. Available online: https://ictv.global/taxonomy.
-
- Pierson T.C., Diamond M.S. Flaviviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 6th ed. Volume 1. Wolters Kluwer Health Adis (ESP); Philadelphia, PA, USA: 2013. p. 2664.
Grants and funding
- 2022/03645-1/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2022/09229-0/Fundação de Amparo à Pesquisa do Estado de São Paulo
- FINEP 01.20.0029.000462/20/The Rede Corona-Ômica BR MCTI/FINEP
- 405786/2022-0/Institutos Nacionais de Ciência e Tecnologia
- 404096/2020-4/National Council for Scientific and Technological Development
LinkOut - more resources
Full Text Sources

