Rapid Development of Modified Vaccinia Virus Ankara (MVA)-Based Vaccine Candidates Against Marburg Virus Suitable for Clinical Use in Humans
- PMID: 39771978
- PMCID: PMC11680136
- DOI: 10.3390/vaccines12121316
Rapid Development of Modified Vaccinia Virus Ankara (MVA)-Based Vaccine Candidates Against Marburg Virus Suitable for Clinical Use in Humans
Abstract
Background/objectives: Marburg virus (MARV) is the etiological agent of Marburg Virus Disease (MVD), a rare but severe hemorrhagic fever disease with high case fatality rates in humans. Smaller outbreaks have frequently been reported in countries in Africa over the last few years, and confirmed human cases outside Africa are, so far, exclusively imported by returning travelers. Over the previous years, MARV has also spread to non-endemic African countries, demonstrating its potential to cause epidemics. Although MARV-specific vaccines are evaluated in preclinical and clinical research, none have been approved for human use. Modified Vaccinia virus Ankara (MVA), a well-established viral vector used to generate vaccines against emerging pathogens, can deliver multiple antigens and has a remarkable clinical safety and immunogenicity record, further supporting its evaluation as a vaccine against MARV. The rapid availability of safe and effective MVA-MARV vaccine candidates would expand the possibilities of multi-factored intervention strategies in endemic countries.
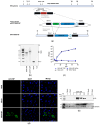
Methods: We have used an optimized methodology to rapidly generate and characterize recombinant MVA candidate vaccines that meet the quality requirements to proceed to human clinical trials. As a proof-of-concept for the optimized methodology, we generated two recombinant MVAs that deliver either the MARV glycoprotein (MVA-MARV-GP) or the MARV nucleoprotein (MVA-MARV-NP).
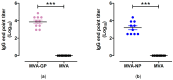
Results: Infections of human cell cultures with recombinant MVA-MARV-GP and MVA-MARV-NP confirmed the efficient synthesis of MARV-GP and MARV-NP proteins in mammalian cells, which are non-permissive for MVA replication. Prime-boost immunizations in C57BL/6J mice readily induced circulating serum antibodies binding to recombinant MARV-GP and MARV-NP proteins. Moreover, the MVA-MARV-candidate vaccines elicited MARV-specific T-cell responses in C57BL/6J mice.
Conclusions: We confirmed the suitability of our two backbone viruses MVA-mCherry and MVA-GFP in a proof-of-concept study to rapidly generate candidate vaccines against MARV. However, further studies are warranted to characterize the protective efficacy of these recombinant MVA-MARV vaccines in other preclinical models and to evaluate them as vaccine candidates in humans.
Keywords: Marburg virus; Modified Vaccinia virus Ankara (MVA); emerging viruses; rapid vaccine development; viral vector vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Eneh S.C., Okonji O.C., Chiburoma A.G., Francisca Ogochukwu O., Tuwleh L., Gideon I., Okonji E.F., Bushabu F.N., Mgbere O. Marburg virus disease amid COVID-19 in West Africa: An emerging and re-emerging zoonotic epidemic threat, future implications and way forward. Ther. Adv. Infect. Dis. 2023;10:20499361231168520. doi: 10.1177/20499361231168520. - DOI - PMC - PubMed
-
- Okonji O.C., Okonji E.F., Mohanan P., Babar M.S., Saleem A., Khawaja U.A., Essar M.Y., Hasan M.M. Marburg virus disease outbreak amidst COVID-19 in the Republic of Guinea: A point of contention for the fragile health system? Clin. Epidemiol. Glob. Health. 2021;13:100920. doi: 10.1016/j.cegh.2021.100920. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

