Respiratory Syncytial Virus: A WAidid Consensus Document on New Preventive Options
- PMID: 39771979
- PMCID: PMC11679680
- DOI: 10.3390/vaccines12121317
Respiratory Syncytial Virus: A WAidid Consensus Document on New Preventive Options
Erratum in
-
Correction: Riccò et al. Respiratory Syncytial Virus: A WAidid Consensus Document on New Preventive Options. Vaccines 2024, 12, 1317.Vaccines (Basel). 2025 Aug 20;13(8):878. doi: 10.3390/vaccines13080878. Vaccines (Basel). 2025. PMID: 40872974 Free PMC article.
Abstract
Background/Objectives: Respiratory syncytial virus (RSV) is a leading cause of respiratory infections, particularly affecting young infants, older adults, and individuals with comorbidities. Methods: This document, developed as a consensus by an international group of experts affiliated with the World Association of Infectious Diseases and Immunological Disorders (WAidid), focuses on recent advancements in RSV prevention, highlighting the introduction of monoclonal antibodies (mAbs) and vaccines. Results: Historically, RSV treatment options were limited to supportive care and the monoclonal antibody palivizumab, which required multiple doses. Recent innovations have led to the development of long-acting mAbs, such as nirsevimab, which provide season-long protection with a single dose. Nirsevimab has shown high efficacy in preventing severe RSV-related lower respiratory tract infections (LRTIs) in infants, reducing hospitalizations and ICU admissions. Additionally, new vaccines, such as RSVpreF and RSVpreF3, target older adults and have demonstrated significant efficacy in preventing LRTIs in clinical trials. Maternal vaccination strategies also show promise in providing passive immunity to newborns, protecting them during the most vulnerable early months of life. This document further discusses the global burden of RSV, its economic impact, and the challenges of implementing these preventative strategies in different healthcare settings. Conclusions: The evidence supports the integration of both passive (mAbs) and active (vaccines) immunization approaches as effective tools to mitigate the public health impact of RSV. The combined use of these interventions could substantially reduce RSV-related morbidity and mortality across various age groups and populations, emphasizing the importance of widespread immunization efforts.
Keywords: RSV; RSV vaccine; lower respiratory tract infections; maternal immunization; monoclonal antibodies; nirsevimab.
Conflict of interest statement
BA received an honorarium for participating in live meetings from Sanofi Pasteur France and Canada related to pertussis and RSV. BA received a nominal payment as a member of a data and safety monitoring board for a study conducted by Chulalongkorn University (Bangkok, Thailand). BA is a co-investigator on studies funded by GSK, Pfizer, Merck, Moderna, Vaccitech, and Inventprise. All funds were paid to his institute, and he did not receive any personal payments. SE received an honorarium for participating in live meetings from Sanofi Pasteur and Pfizer. SE is a co-investigator on a study funded by Merck. All funds were paid to her institute, and she did not receive any personal payments. The other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures

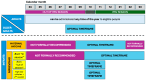
References
-
- Shi T., Denouel A., Tietjen A.K., Campbell I., Moran E., Li X., Campbell H., Demont C., Nyawanda B.O., Chu H.Y., et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2021;222:S577–S583. doi: 10.1093/infdis/jiz059. - DOI - PubMed
-
- Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., Madhi S.A., Omer S.B., Simões E.A.F., Campbell H., et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger than 5 Years in 2019: A Systematic Analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

