RSV Vaccine with Nanoparticle-Based Poly-Sorbitol Transporter (PST) Adjuvant Improves Respiratory Protection Against RSV Through Inducing Both Systemic and Mucosal Humoral Immunity
- PMID: 39772016
- PMCID: PMC11680183
- DOI: 10.3390/vaccines12121354
RSV Vaccine with Nanoparticle-Based Poly-Sorbitol Transporter (PST) Adjuvant Improves Respiratory Protection Against RSV Through Inducing Both Systemic and Mucosal Humoral Immunity
Abstract
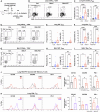
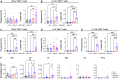
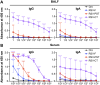
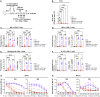
Background/Objectives: Respiratory syncytial virus (RSV) causes symptoms similar to a mild cold for adults, but in case of infants, it causes bronchitis and/or pneumonia, and in some cases, mortality. Mucosal immunity within the respiratory tract includes tissue-resident memory T (TRM) cells and tissue-resident memory B (BRM) cells, which provides rapid and efficient protection against RSV re-infection. Therefore, vaccine strategies should aim to generate mucosal immune responses. However, the interactions between RSV vaccines and mucosal immune responses within the respiratory tract are poorly understood. We evaluated a mucosal immune system following immunization by RSV vaccine with poly-sorbitol transporter (RSV-PST), a nanoparticle adjuvant. Methods: We intranasally immunized the RSV-PST and identified the systemic and mucosal immune responses. Furthermore, we challenged with RSV A2 strain after immunization and investigated the protective effects. Results: Consequently, antigen-specific CD8+ TRM cells were markedly elevated in the lung parenchyma, yet exhibited impaired cytokine expression. In contrast, humoral immunity, with systemic antibody production from serum, but not in the respiratory tract, was significantly increased by RSV-PST immunization. Interestingly, the production of respiratory mucosal antigen-specific IgG after RSV A2 challenge dramatically increased in the bronchoalveolar lavage fluid (BALF) of the RSV-PST immunized group in the presence of FTY720, and the lung-infected RSV titer was significantly lower in this group. Furthermore, after RSV A2 challenge, CD69+ IgG+ BRM cells were significantly increased in lung tissues in the RSV-PST group. Conclusions: The RSV-PST vaccine has protective effects against RSV infection by promoting both systemic and local humoral immunity rather than cellular immunity.
Keywords: adjuvant; mucosal immunity; nanovaccine; poly-sorbitol transporter (PST); respiratory syncytial virus (RSV); systemic humoral immune response; vaccination.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Mucosal immunization with an adenoviral vector vaccine confers superior protection against RSV compared to natural immunity.Front Immunol. 2022 Jul 28;13:920256. doi: 10.3389/fimmu.2022.920256. eCollection 2022. Front Immunol. 2022. PMID: 36003372 Free PMC article.
-
Intranasal Inoculation of Cationic Crosslinked Carbon Dots-Adjuvanted Respiratory Syncytial Virus F Subunit Vaccine Elicits Mucosal and Systemic Humoral and Cellular Immunity.MedComm (2020). 2025 Mar 24;6(4):e70146. doi: 10.1002/mco2.70146. eCollection 2025 Apr. MedComm (2020). 2025. PMID: 40135196 Free PMC article.
-
Filling two needs with one deed: a combinatory mucosal vaccine against influenza A virus and respiratory syncytial virus.Front Immunol. 2024 Jun 21;15:1376395. doi: 10.3389/fimmu.2024.1376395. eCollection 2024. Front Immunol. 2024. PMID: 38975350 Free PMC article.
-
The third pandemic: The respiratory syncytial virus landscape and specific considerations for the allergist/immunologist.Allergy Asthma Proc. 2023 Jul 26;44(4):220-228. doi: 10.2500/aap.2023.44.230030. Allergy Asthma Proc. 2023. PMID: 37236777 Review.
-
Induction and Subversion of Human Protective Immunity: Contrasting Influenza and Respiratory Syncytial Virus.Front Immunol. 2018 Mar 2;9:323. doi: 10.3389/fimmu.2018.00323. eCollection 2018. Front Immunol. 2018. PMID: 29552008 Free PMC article. Review.
References
-
- Zhang L., Peeples M.E., Boucher R.C., Collins P.L., Pickles R.J. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 2002;76:5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. - DOI - PMC - PubMed
-
- Guo-Parke H., Canning P., Douglas I., Villenave R., Heaney L.G., Coyle P.V., Lyons J.D., Shields M.D., Power U.F. Relative respiratory syncytial virus cytopathogenesis in upper and lower respiratory tract epithelium. Am. J. Respir. Crit. Care Med. 2013;188:842–851. doi: 10.1164/rccm.201304-0750OC. - DOI - PubMed
-
- Wang X., Li Y., Shi T., Bont L.J., Chu H.Y., Zar H.J., Wahi-Singh B., Ma Y., Cong B., Sharland E., et al. Global disease burden of and risk factors for acute lower respiratory infections caused by respiratory syncytial virus in preterm infants and young children in 2019: A systematic review and meta-analysis of aggregated and individual participant data. Lancet. 2024;403:1241–1253. doi: 10.1016/S0140-6736(24)00138-7. - DOI - PubMed
-
- Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., Madhi S.A., Omer S.B., Simoes E.A.F., Campbell H., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

