A Preclinical Immunogenicity Study of the Recombinant Human Papillomavirus Nine-Valent Virus-like Particle Vaccine
- PMID: 39772018
- PMCID: PMC11680239
- DOI: 10.3390/vaccines12121356
A Preclinical Immunogenicity Study of the Recombinant Human Papillomavirus Nine-Valent Virus-like Particle Vaccine
Abstract
Background: Cervical cancer is associated with persistent infection of high-risk human papillomaviruses (HPVs). Prophylactic HPV vaccines have been recommended and have significant efficacy in preventing cervical cancer. Multivalent HPV vaccines have a better preventative effect on HPV-related diseases. However, there is currently only one nine-valent HPV vaccine on the market: Gardasil® 9. The development of new HPV vaccines is still urgent in order to achieve the goal of eliminating cervical cancer as proposed by the WHO.
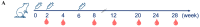
Methods: In this study, we developed a nine-valent recombinant HPV virus-like particle (VLP) vaccine (HPV-9 vaccine) containing HPV type 6, 11, 16, 18, 31, 33, 45, 52, and 58 antigens, with an adjuvant of aluminum phosphate (AlPO4). The type-specific L1 proteins were recombinantly expressed using Pichia pastoris, followed by self-assembly into VLPs. Immunogenicity studies of the HPV-9 vaccine were performed using rodents (mice and rats) and non-human primates (macaques) as animal models.
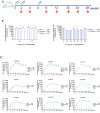
Results: Immunogenicity studies showed that the HPV-9 vaccine is able to elicit a robust and long-lasting neutralizing antibody response in rodents (mice and rats) and non-human primates (cynomolgus macaque) models. The HPV-9 vaccine shows immunogenicity comparable to that of Walrinvax® and Gardasil® 9.
Conclusions: In summary, this study provides a comprehensive investigation of the immunogenicity of the HPV-9 vaccine, including its immune persistence. These findings, derived from using models of diverse animal species, contribute valuable insights into the potential efficacy of the vaccine candidate in clinical settings.
Keywords: human papillomavirus (HPV); immunogenicity; protective efficacy; vaccines; virus-like particles (VLPs).
Conflict of interest statement
D.X., J.-D.L., J.A., X.-X.M., X.-L.W., Z.Z., H.-P.L., M.-J.D., Y.-X.J., L.-Y.Z., and C.-L.Z. are employees of Shanghai Zerun Biotech Co., Ltd. X.T. is a former employee of Shanghai Zerun Biotech Co., Ltd. and currently works at Yunnan Walvax Biotech Co., Ltd., which is the holding company of Shanghai Zerun Biotech Co., Ltd.
Figures


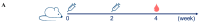
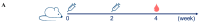


Similar articles
-
Broad Cross-Protection Is Induced in Preclinical Models by a Human Papillomavirus Vaccine Composed of L1/L2 Chimeric Virus-Like Particles.J Virol. 2016 Jun 24;90(14):6314-25. doi: 10.1128/JVI.00449-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147749 Free PMC article.
-
Immunogenicity of next-generation HPV vaccines in non-human primates: Measles-vectored HPV vaccine versus Pichia pastoris recombinant protein vaccine.Vaccine. 2016 Sep 7;34(39):4724-4731. doi: 10.1016/j.vaccine.2016.07.051. Epub 2016 Aug 11. Vaccine. 2016. PMID: 27523740 Free PMC article.
-
N-terminal truncations on L1 proteins of human papillomaviruses promote their soluble expression in Escherichia coli and self-assembly in vitro.Emerg Microbes Infect. 2018 Sep 26;7(1):160. doi: 10.1038/s41426-018-0158-2. Emerg Microbes Infect. 2018. PMID: 30254257 Free PMC article.
-
Prophylactic vaccines against HPV-caused cervical cancer: novel vaccines are still demanded.Infect Agent Cancer. 2025 Mar 10;20(1):16. doi: 10.1186/s13027-025-00643-5. Infect Agent Cancer. 2025. PMID: 40059217 Free PMC article. Review.
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
References
-
- Cervical Cancer. [(accessed on 17 November 2020)]. Available online: https://www.who.int/health-topics/cervical-cancer#tab=tab_2.
Grants and funding
LinkOut - more resources
Full Text Sources

