The Protective Effect of IL-17A in Pneumonic Plague Can Be Compensated by Effective Vaccines and Immunization Strategies in Mice
- PMID: 39772023
- PMCID: PMC11680114
- DOI: 10.3390/vaccines12121361
The Protective Effect of IL-17A in Pneumonic Plague Can Be Compensated by Effective Vaccines and Immunization Strategies in Mice
Abstract
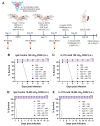
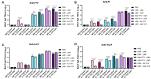
Plague, caused by Yersinia pestis, poses a public health threat not only due to sporadic outbreaks across the globe but also due to its potential as a biothreat agent. Ironically, among the seven deadliest pandemics in global history, three were caused by Y. pestis. Pneumonic plague, the more contagious and severe form of the disease, is difficult to contain, requiring either prophylactic antibiotic treatment or vaccination. However, no vaccine (live attenuated or subunit) is currently approved by the Food and Drug Administration, requiring rigorous preclinical studies in different animal models, thus forming the basis of this study. Objectives: The aim of this study was to evaluate the efficacy and immune responses of two live attenuated vaccines (LAVs), LMA and LMP, either alone or in combination with a trivalent adenoviral vector-based vaccine (Ad5-YFV), in IL-17A-depleted and IgG control mice by using an anti-IL-17A monoclonal antibody (mAb) or its matched isotype IgG, respectively. Methods: IL-17A mAb or IgG isotype control was administered to mice twice per week to their respective groups during the course of immunization. Serum, spleens, and broncho-alveolar lavage fluid (BALF) were collected for assessing immunological responses, and another cohort of mice was intranasally challenged with a lethal dose of parental Y. pestis CO92. Results: Robust humoral and cellular immune responses followed by complete protection were observed in all vaccinated animals against highly lethal intranasal challenge doses of parental Y. pestis CO92. Serum IgG titers to YscF and overall mucosal IgA titers to all three antigens of the Ad5-YFV vaccine were significantly lower, with slightly reduced serum LcrV-neutralizing antibodies when IL-17A was depleted compared to IgG control animals during the course of immunization. A remarkable reduction in Th1 (IFNγ or IL-2) and Th17 cell populations was observed in IL-17A-depleted mice compared to IgG controls in response to vaccination. On the other hand, B cell activities in germinal centers, overall activated antigen-specific T cells, and memory B and T cells remained at comparable levels in both vaccinated IL-17A-depleted and IgG control mice. Conclusions: These data demonstrated the effectiveness of our vaccines even under the reduced levels of both Th1 and Th17 responses and thus should be suitable for those individuals associated with certain immune deficiencies.
Keywords: IL-17A-depleted mice; Y. pestis; flow cytometry; homologous and heterologous vaccinations; immunogenicity; live attenuated and adenovirus-based plague vaccines; pneumonic plague.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Combination of live attenuated and adenovirus-based vaccines completely protects interferon gamma (IFNγ) knockout mice against pneumonic plague.bioRxiv [Preprint]. 2024 Dec 11:2024.12.06.627261. doi: 10.1101/2024.12.06.627261. bioRxiv. 2024. PMID: 39713400 Free PMC article. Preprint.
-
A bacteriophage cocktail targeting Yersinia pestis provides strong post-exposure protection in a rat pneumonic plague model.Microbiol Spectr. 2024 Nov 5;12(11):e0094224. doi: 10.1128/spectrum.00942-24. Epub 2024 Sep 18. Microbiol Spectr. 2024. PMID: 39292000 Free PMC article.
-
Nanolipoprotein particle (NLP) vaccine confers protection against Yersinia pestis aerosol challenge in a BALB/c mouse model.Front Immunol. 2025 Jun 26;16:1603710. doi: 10.3389/fimmu.2025.1603710. eCollection 2025. Front Immunol. 2025. PMID: 40642079 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Feodorova V.A., Lyapina A.M., Khizhnyakova M.A., Zaitsev S.S., Sayapina L.V., Arseneva T.E., Trukhachev A.L., Lebedeva S.A., Telepnev M.V., Ulianova O.V., et al. Humoral and cellular immune responses to Yersinia pestis Pla antigen in humans immunized with live plague vaccine. PLoS Neglected Trop. Dis. 2018;12:e0006511. doi: 10.1371/journal.pntd.0006511. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

