Evaluation of Safety, Immunogenicity and Cross-Reactive Immunity of OVX836, a Nucleoprotein-Based Universal Influenza Vaccine, in Older Adults
- PMID: 39772052
- PMCID: PMC11728545
- DOI: 10.3390/vaccines12121391
Evaluation of Safety, Immunogenicity and Cross-Reactive Immunity of OVX836, a Nucleoprotein-Based Universal Influenza Vaccine, in Older Adults
Abstract
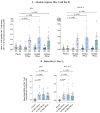
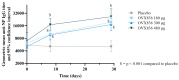
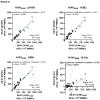
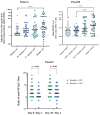
Background/Objectives: In a Phase 2a, double-blind, placebo-controlled study including healthy participants aged 18-55 years, OVX836, a nucleoprotein (NP)-based candidate vaccine, previously showed a good safety profile, a robust immune response (both humoral and cellular) and a preliminary signal of protection (VE = 84%) against PCR-confirmed symptomatic influenza after a single intramuscular dose of 180 µg, 300 µg or 480 µg. Methods: Using the same methodology, we confirmed the good safety and strong immunogenicity of OVX836 at the same doses in older adults (≥65 years), a key target population for influenza vaccination. Results: Significant humoral (anti-NP IgG) and cellular (interferon gamma (IFNγ) spot-forming cells per million peripheral blood mononuclear cells and specific CD4+ IFNγ+ T-cells) immune responses were observed at the three dose levels, without clear dose-response relationship. T-cell responses were shown to be highly cross-reactive against various influenza A strains, both seasonal and highly pathogenic avian strains. We also evaluated the effect of sex (stronger immune response in females) and age (stronger immune response in young adults) on the immune response to OVX836 after adjustment based on the pre-vaccination immune status. Conclusions: The results obtained with OVX836 lay the groundwork for a future placebo-controlled, field proof of concept efficacy Phase 2b trial.
Keywords: Phase 2a; cross-reactivity; healthy participants; immunogenicity; influenza; older adults; safety; universal vaccine.
Conflict of interest statement
Nicola Groth, Jessika Tourneur, Florence Nicolas and Alexandre Le Vert are employees and shareholders of OSIVAX. Jacques Bruhwyler, Philippe Moris and Geert Leroux-Roels received consulting honoraria from OSIVAX. Isabel Leroux-Roels, Gwenn Waerlop, Yorick Janssens, Fien De Boever, Azhar Alhatemi and Bart Jacobs, all affiliated with CEVAC (Ghent University Hospital and Ghent University), conducted the clinical trial sponsored by OSIVAX, for which their institution received financial compensation.
Figures



References
-
- Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., Cohen C., Gran J.M., Schanzer D., Cowling B.J., et al. Estimates of Global Seasonal Influenza-Associated Respiratory Mortality: A Modelling Study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. - DOI - PMC - PubMed
-
- Lafond K.E., Porter R.M., Whaley M.J., Suizan Z., Ran Z., Aleem M.A., Thapa B., Sar B., Proschle V.S., Peng Z., et al. Global Burden of Influenza-Associated Lower Respiratory Tract Infections and Hospitalizations among Adults: A Systematic Review and Meta-Analysis. PLoS Med. 2021;18:e1003550. doi: 10.1371/journal.pmed.1003550. - DOI - PMC - PubMed
-
- Rose A., Kissling E., Emborg H.-D., Larrauri A., McMenamin J., Pozo F., Trebbien R., Mazagatos C., Whitaker H., Valenciano M., et al. Interim 2019/20 Influenza Vaccine Effectiveness: Six European Studies, September 2019 to January 2020. Eurosurveillance. 2020;25:2000153. doi: 10.2807/1560-7917.ES.2020.25.10.2000153. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

