Seroprevalence of Antibodies to Filoviruses with Outbreak Potential in Sub-Saharan Africa: A Systematic Review to Inform Vaccine Development and Deployment
- PMID: 39772055
- PMCID: PMC11726543
- DOI: 10.3390/vaccines12121394
Seroprevalence of Antibodies to Filoviruses with Outbreak Potential in Sub-Saharan Africa: A Systematic Review to Inform Vaccine Development and Deployment
Abstract
Background/Objectives: Orthoebolaviruses and orthomarburgviruses are filoviruses that can cause viral hemorrhagic fever and significant morbidity and mortality in humans. The evaluation and deployment of vaccines to prevent and control Ebola and Marburg outbreaks must be informed by an understanding of the transmission and natural history of the causative infections, but little is known about the burden of asymptomatic infection or undiagnosed disease. This systematic review of the published literature examined the seroprevalence of antibodies to orthoebolaviruses and orthomarburgviruses in sub-Saharan Africa. Methods: The review protocol was registered on PROSPERO (ID: CRD42023415358) and previously published. Eighty-seven articles describing 85 studies were included, of which seventy-six measured antibodies to orthoebolaviruses and forty-one measured antibodies to orthomarburgviruses. Results: The results highlight three central findings that may have implications for vaccine development and deployment. First, substantial antibody seropositivity to Ebola virus (EBOV) and Sudan virus (SUDV) was observed in populations from outbreak-affected areas (≤33% seroprevalence among general populations; ≤41% seroprevalence among healthcare workers and close contacts of disease cases). Second, antibody seropositivity to EBOV, SUDV, and Marburg virus (MARV) was observed among populations from areas without reported outbreaks, with seroprevalence ranging from <1 to 21%. Third, in Central and East Africa, MARV antibody seroprevalence was substantially lower than EBOV or SUDV antibody seroprevalence, even in outbreak-affected areas and in populations at a moderate or high risk of infection (with MARV seroprevalence mostly ranging from 0 to 3%). Conclusions: Whilst gaps remain in our understanding of the significance of antibody seropositivity in some settings and contexts, these findings may be important in considering target indications for novel filovirus vaccines, in defining study designs and strategies for demonstrating vaccine efficacy or effectiveness, and in planning and evaluating vaccine deployment strategies to prevent and control outbreaks.
Keywords: filoviruses; human infection; seroprevalence; sub-Saharan Africa; systematic review.
Conflict of interest statement
The authors declare that they have no direct financial interest or personal relationships that could influence the content of this manuscript. However, it is important to note that IAVI is actively engaged in the research and development of filovirus vaccines, which may be relevant to the topics covered in the systematic review. Some of the authors are currently employed by IAVI, while others are previously employed with IAVI. This work is funded by BARDA and the affiliation may be considered potential conflicts of interest.
Figures
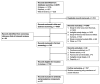
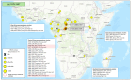






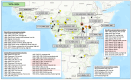


References
-
- Feldmann H., Klank H.-D. Filoviruses. In: Baron S., editor. Medical Microbiology. Volume 4 University of Texas Medical Branch at Galveston; Galveston, TX, USA: 1996.
-
- Biedenkopf N., Bukreyev A., Chandran K., Di Paola N., Formenty P.B.H., Griffiths A., Hume A.J., Muhlberger E., Netesov S.V., Palacios G., et al. Renaming of genera Ebolavirus and Marburgvirus to Orthoebolavirus and Orthomarburgvirus, respectively, and introduction of binomial species names within family Filoviridae. Arch. Virol. 2023;168:220. doi: 10.1007/s00705-023-05834-2. - DOI - PubMed
-
- Ebola Virus Disease. [(accessed on 10 October 2024)]. Available online: https://www.paho.org/en/topics/ebola-virus-disease#:~:text=The%20average....
-
- Marburg Virus Disease. [(accessed on 10 October 2024)]. Available online: https://www.who.int/health-topics/marburg-virus-disease#tab=tab_1.
-
- Marburg Virus Disease—Ghana. [(accessed on 10 October 2024)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON409.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

