Safety, Immunogenicity, and Efficacy of a Recombinant Vesicular Stomatitis Virus Vectored Vaccine Against Severe Fever with Thrombocytopenia Syndrome Virus and Heartland Bandavirus
- PMID: 39772063
- PMCID: PMC11728676
- DOI: 10.3390/vaccines12121403
Safety, Immunogenicity, and Efficacy of a Recombinant Vesicular Stomatitis Virus Vectored Vaccine Against Severe Fever with Thrombocytopenia Syndrome Virus and Heartland Bandavirus
Abstract
Background: Severe fever with thrombocytopenia syndrome virus (SFTSV) is a recently emerged tickborne virus in east Asia with over 18,000 confirmed cases. With a high case fatality ratio, SFTSV has been designated a high priority pathogen by the WHO and the NIAID. Despite this, there are currently no approved therapies or vaccines to treat or prevent SFTS. Vesicular stomatitis virus (VSV) represents an FDA-approved vaccine platform that has been considered for numerous viruses due to its low sero-prevalence in humans, ease in genetic manipulation, and promiscuity in incorporating foreign glycoproteins into its virions.
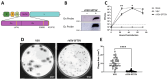
Methods: In this study, we developed a recombinant VSV (rVSV) expressing the SFTSV glycoproteins Gn/Gc (rVSV-SFTSV) and assessed its safety, immunogenicity, and efficacy in C57BL/6, Ifnar-/-, and AG129 mice.
Results: We demonstrate that rVSV-SFTSV is safe when given to immunocompromised animals and is not neuropathogenic when injected intracranially into young immunocompetent mice. Immunization of wild type (C57BL/6) and Ifnar-/- mice with rVSV-SFTSV resulted in high levels of neutralizing antibodies and protection in a lethal SFTSV challenge model. Additionally, passive transfer of sera from immunized Ifnar-/- mice into naïve animals was protective when given pre- or post-exposure. Finally, we demonstrate that immunization with rVSV-SFTSV cross protects AG129 mice against challenge with the closely related Heartland bandavirus despite negligible neutralizing titers to the virus.
Conclusions: Taken together, these data suggest that rVSV-SFTSV is a promising vaccine candidate for SFTSV and Heartland bandavirus with a favorable safety profile.
Keywords: Dabie bandavirus; bandavirus; heartland bandavirus; heartland virus; immunity; neutralizing antibodies; rVSV; severe fever with thrombocytopenia syndrome virus; tick-borne virus; vaccine; vesicular stomatitis virus.
Conflict of interest statement
Authors hereby declare that there are no conflicts of interest in the manuscript.
Figures

Similar articles
-
Molecular mechanism of potently neutralizing human monoclonal antibodies against severe fever with thrombocytopenia virus infection.J Virol. 2025 Jul 22;99(7):e0053325. doi: 10.1128/jvi.00533-25. Epub 2025 Jun 20. J Virol. 2025. PMID: 40539781 Free PMC article.
-
Mucosal Delivery of Recombinant Vesicular Stomatitis Virus Vectors Expressing Envelope Proteins of Respiratory Syncytial Virus Induces Protective Immunity in Cotton Rats.J Virol. 2021 Feb 24;95(6):e02345-20. doi: 10.1128/JVI.02345-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33408176 Free PMC article.
-
Two Point Mutations in the Glycoprotein of SFTSV Enhance the Propagation Recombinant Vesicular Stomatitis Virus Vectors at Assembly Step.Viruses. 2023 Mar 21;15(3):800. doi: 10.3390/v15030800. Viruses. 2023. PMID: 36992507 Free PMC article.
-
A Brighton Collaboration standardized template with key considerations for a benefit/risk assessment for the emergent vesicular stomatitis virus (VSV) viral vector vaccine for Lassa fever.Vaccine. 2025 Jun 11;58:127137. doi: 10.1016/j.vaccine.2025.127137. Epub 2025 May 13. Vaccine. 2025. PMID: 40367816 Review.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
References
-
- Cui H., Shen S., Chen L., Fan Z., Wen Q., Xing Y., Wang Z., Zhang J., Chen J., La B., et al. Global epidemiology of severe fever with thrombocytopenia syndrome virus in human and animals: A systematic review and meta-analysis. Lancet Reg. Health West. Pac. 2024;48:101133. doi: 10.1016/j.lanwpc.2024.101133. - DOI - PMC - PubMed
-
- Takahashi T., Maeda K., Suzuki T., Ishido A., Shigeoka T., Tominaga T., Kamei T., Honda M., Ninomiya D., Sakai T., et al. The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J. Infect. Dis. 2014;209:816–827. doi: 10.1093/infdis/jit603. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

