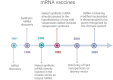
mRNA Vaccines Against COVID-19 as Trailblazers for Other Human Infectious Diseases
- PMID: 39772079
- PMCID: PMC11680146
- DOI: 10.3390/vaccines12121418
mRNA Vaccines Against COVID-19 as Trailblazers for Other Human Infectious Diseases
Abstract
mRNA vaccines represent a milestone in the history of vaccinology, because they are safe, very effective, quick and cost-effective to produce, easy to adapt should the antigen vary, and able to induce humoral and cellular immunity.
Methods: To date, only two COVID-19 mRNA and one RSV vaccines have been approved. However, several mRNA vaccines are currently under development for the prevention of human viral (influenza, human immunodeficiency virus [HIV], Epstein-Barr virus, cytomegalovirus, Zika, respiratory syncytial virus, metapneumovirus/parainfluenza 3, Chikungunya, Nipah, rabies, varicella zoster virus, and herpes simplex virus 1 and 2), bacterial (tuberculosis), and parasitic (malaria) diseases.
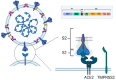
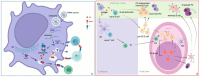
Results: RNA viruses, such as severe acute respiratory syndrome coronavirus (SARS-CoV)-2, HIV, and influenza, are characterized by high variability, thus creating the need to rapidly adapt the vaccines to the circulating viral strain, a task that mRNA vaccines can easily accomplish; however, the speed of variability may be higher than the time needed for a vaccine to be adapted. mRNA vaccines, using lipid nanoparticles as the delivery system, may act as adjuvants, thus powerfully stimulating innate as well as adaptive immunity, both humoral, which is rapidly waning, and cell-mediated, which is highly persistent. Safety profiles were satisfactory, considering that only a slight increase in prognostically favorable anaphylactic reactions in young females and myopericarditis in young males has been observed.
Conclusions: The COVID-19 pandemic determined a shift in the use of RNA: after having been used in medicine as micro-RNAs and tumor vaccines, the new era of anti-infectious mRNA vaccines has begun, which is currently in great development, to either improve already available, but unsatisfactory, vaccines or develop protective vaccines against infectious agents for which no preventative tools have been realized yet.
Keywords: COVID-19; infectious diseases; mRNA; vaccines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Melton D.A., Krieg P.A., Rebagliati M.R., Maniatis T., Zinn K., Green M.R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984;12:7035–7056. doi: 10.1093/nar/12.18.7035. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

