A Trivalent Live Vaccine Elicits Cross-Species Protection Against Acute Otitis Media in a Murine Model
- PMID: 39772092
- PMCID: PMC11728825
- DOI: 10.3390/vaccines12121432
A Trivalent Live Vaccine Elicits Cross-Species Protection Against Acute Otitis Media in a Murine Model
Abstract
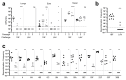
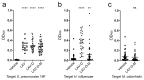
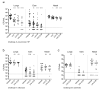
Background: Acute otitis media (AOM) is a common pediatric infection worldwide and is the primary basis for pediatric primary care visits and antibiotic prescriptions in children. Current licensed vaccines have been incompletely ineffective at reducing the global burden of AOM, underscoring a major unmet medical need. The complex etiology of AOM presents additional challenges for vaccine development, as it can stem from multiple bacterial species including Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. As such, targeting multiple pathogens simultaneously may be required to significantly impact the overall disease burden. Methods: In this study, we aim to overcome this challenge by engineering a live-attenuated vaccine platform based on an attenuated mutant of S. pneumoniae that expresses H. influenzae and M. catarrhalis surface epitopes to induce protective immunity against all three pathogens. Results: The trivalent live-attenuated vaccine conferred significant protection against all three bacterial otopathogens as measured by seroconversion and the development of AOM, with the inclusion of the additional epitopes providing unexpected synergy and enhanced protection against S. pneumoniae. Conclusions: These data demonstrate a novel mechanism of introducing non-native immunogenic antigens into a live-attenuated vaccine platform to engender protection against AOM from multiple pathogenic species.
Keywords: acute otitis media; live-attenuated vaccine; multiple otopathogens.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Preventing and Treating Ear Infections. [(accessed on 11 December 2024)];2017 Available online: https://www.cdc.gov/ear-infection/media/pdfs/Ear-Infection-508.pdf.
-
- Block S.L., Hedrick J., Harrison C.J., Tyler R., Smith A., Findlay R., Keegan E. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr. Infect. Dis. J. 2004;23:829–833. doi: 10.1097/01.inf.0000136868.91756.80. - DOI - PubMed
Grants and funding
- NIAID 1R21AI178085-01A1, NIAID 1R21AI178084-01A, NIAID U19AI158076, NIAID 1R01AI155614, NIAID 1R01AI171038, NIAID R01AI156898/AI/NIAID NIH HHS/United States
- R56 AI155614/AI/NIAID NIH HHS/United States
- R01 AI171038/AI/NIAID NIH HHS/United States
- U19 AI158076/AI/NIAID NIH HHS/United States
- R21 AI178084/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

