Construction of the First Russian Recombinant Live Attenuated Vaccine Strain and Evaluation of Its Protection Efficacy Against Two African Swine Fever Virus Heterologous Strains of Serotype 8
- PMID: 39772103
- PMCID: PMC11680325
- DOI: 10.3390/vaccines12121443
Construction of the First Russian Recombinant Live Attenuated Vaccine Strain and Evaluation of Its Protection Efficacy Against Two African Swine Fever Virus Heterologous Strains of Serotype 8
Abstract
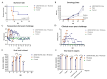
Background/Objectives: The spread of African swine fever virus (ASFV) has led to major economic losses to pork worldwide. In Russia, there are no developed or registered vaccines against ASFV genotype II, which is associated with numerous ASFV outbreaks in populations of domestic pigs and wild boars in the country. Methods: We introduced deletions of the six MGF360 and MGF505 genes of the ASFV virulent Stavropol_01/08 strain, isolated in Russia in 2008. Results: We show here that this deletion did lead to full attenuation of the ASFV virulent Stavropol_01/08 strain. Animals intramuscularly inoculated with 104 HAD50 of ΔMGF360/505_Stav developed a strong immune response and short period of viremia (at 3-7 days post-inoculation). Recombinant ΔMGF360/505_Stav strain provides complete protection of pigs against the ASFV parental Stavropol_01/08 strain (103 HAD50). Therefore, in our experiment, we did not detect the genome of both the virulent and the recombinant strains in the blood and organs post-challenge with the Stavropol_01/08. In contrast, we found only partial protection (40%) of the ΔMGF360/505_Stav-immunized pigs against challenge with the ASFV heterologous Rhodesia strain. Additionally, the surviving animals had a prolonged fever, and their condition was depressed for most of the experiment. Conclusions: Thus, the ASFV recombinant ΔMGF360/505_Stav strain is the first live attenuated vaccine (LAV) in Russia that induces complete protection in pigs challenged with the highly virulent, epidemiologically relevant strains genotype II and serotype 8. However, this ASF LAV is not able to provide a high level of protection against other variants of serotype 8.
Keywords: African swine fever; African swine fever virus; MGF360; MGF505; genotype; live attenuated vaccine; serogroup; serotype.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources

