Boosting Vaccine Research: The 16-Year Journey of TRANSVAC Vaccine Infrastructure
- PMID: 39772106
- PMCID: PMC11680270
- DOI: 10.3390/vaccines12121446
Boosting Vaccine Research: The 16-Year Journey of TRANSVAC Vaccine Infrastructure
Abstract
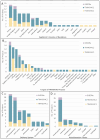
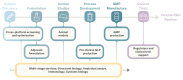
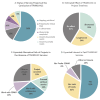
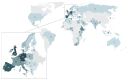
TRANSVAC represents a long-running effort to accelerate the development of novel vaccines by integrating institutions from across Europe under a single collaborative framework. This initiative has empowered the global vaccine community since 2009 including contributing toward the development and optimization of vaccine candidates as well as the provision of new adjuvants, research protocols, and technologies. Scientific services were provided in support of 88 different vaccine development projects, and 400 professionals attended TRANSVAC training events on various vaccine-related topics. Here, we review the accomplishments of the TRANSVAC consortia and analyze the continued needs of academic and industrial vaccine developers in Europe. The findings highlight the benefits of coordination across different sectors, both through research infrastructures such as TRANSVAC and other mechanisms, to address the current and future global health challenges and ensure that European vaccine developers have the support required to successfully compete in the global market.
Keywords: research and development; research infrastructure; vaccine.
Conflict of interest statement
P.V.d.L. is named as a co-inventor in two patents covering the use of modified LPS adjuvants discussed in the text (WO2017129752 and WO2017129761). C.A.G. is named as a co-inventor in a patent covering the use of CDA as a neonatal adjuvant discussed in the text (EP 19193982), which was previously patented covering the use of CDA as an adjuvant (PCT/EP 2006010693). Author P.V.d.L. was employed by the company Intravacc BV. The authors declare no other conflicts of interest.
Figures


References
-
- Geels M.J., Thøgersen R.L., Guzman C.A., Ho M.M., Verreck F., Collin N., Robertson J.S., McConkey S.J., Kaufmann S.H.E., Leroy O. TRANSVAC Research Infrastructure–Results and Lessons Learned from the European Network of Vaccine Research and Development. Vaccine. 2015;33:5481–5487. doi: 10.1016/j.vaccine.2015.01.079. - DOI - PubMed
-
- Meslé M.M.I., Brown J., Mook P., Katz M.A., Hagan J., Pastore R., Benka B., Redlberger-Fritz M., Bossuyt N., Stouten V., et al. Estimated Number of Lives Directly Saved by COVID-19 Vaccination Programmes in the WHO European Region from December, 2020, to March, 2023: A Retrospective Surveillance Study. Lancet Respir. Med. 2024;12:714–727. doi: 10.1016/S2213-2600(24)00179-6. - DOI - PubMed
-
- European Network of Vaccine Development and Research|TRANSVAC|Project|Fact Sheet|FP7|CORDIS|European Commission. [(accessed on 15 October 2024)]. Available online: https://cordis.europa.eu/project/id/228403.
-
- Vaccines Europe Pipeline Review 2023. [(accessed on 15 October 2024)]. Available online: https://www.vaccineseurope.eu/wp-content/uploads/2023/11/VaccinesEurope-....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

