T Cell Responses to BA.2.86 and JN.1 SARS-CoV-2 Variants in Elderly Subjects
- PMID: 39772110
- PMCID: PMC11680353
- DOI: 10.3390/vaccines12121451
T Cell Responses to BA.2.86 and JN.1 SARS-CoV-2 Variants in Elderly Subjects
Abstract
Background/objectives: New SARS-CoV-2 variants are continuously emerging, making it essential to assess the efficacy of vaccine-induced immune protection. Limited information is available regarding T cell responses to BA.2.86 and JN.1 variants, particularly in elderly individuals.
Methods: We evaluated T cell and total IgG responses against the receptor-binding domain (RBD) of the ancestral SARS-CoV-2 strain, as well as BA.2.86 and JN.1 omicron subvariants, in two groups of subjects. One group consisted of SARS-CoV-2-exposed elderly individuals who were fully vaccinated with the BNT162B2 mRNA vaccine, with a booster dose of the updated 2023-2024 COVID-19 vaccine (XBB.1.5) at least 15 days after receiving a booster dose of the updated 2023-2024 COVID-19 vaccine. The second group consisted of healthcare workers who were unexposed to SARS-CoV-2 one month after the booster dose of the first-generation BNT162b2 mRNA vaccine. T cell activation-induced markers (AIM) and IFN-γ secretion were evaluated by flow cytometry and ELISpot assays, respectively.
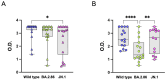
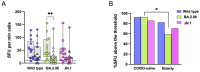
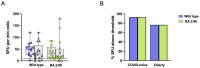
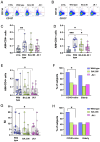
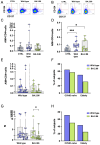
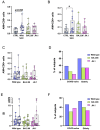
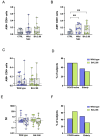
Results: Elderly subjects showed reduced IgG levels against JN.1 compared with the ancestral strain. BA.2.86 stimulation resulted in lower IFN-γ levels in the elderly versus the COVID-19-naïve group. AIM analysis showed that among T cells, CD4+ were the most responsive, with a reduced proportion of JN.1-reactive CD4+ T cells compared with the ancestral strain in the SARS-CoV-2-unexposed group. Despite receiving the updated booster, the elderly group showed reduced CD4+ T cell reactivity to BA.2.86.
Conclusions: The XBB.1.5-containing vaccine induced lower CD4+ T cell responses against BA.2.86 in the elderly. CD4+ T cells from BNT16b2-vaccinated, COVID-19-naïve subjects recognized ancestral and BA.2.86 RBD strains while showing reduced responses to JN.1. These results emphasize the need for tailored vaccine strategies for emerging variants, particularly in vulnerable populations.
Keywords: BA.2.86; COVID-19; JN.1; SARS-CoV-2; elderly; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Omicron BA.1-specific T-cell responses in adults vaccinated with CoronaVac or BNT162b2 in Hong Kong: an observational cohort study.Lancet Microbe. 2023 Jun;4(6):e418-e430. doi: 10.1016/S2666-5247(23)00006-X. Epub 2023 Apr 20. Lancet Microbe. 2023. PMID: 37086735 Free PMC article.
-
Relative vaccine protection, disease severity, and symptoms associated with the SARS-CoV-2 omicron subvariant BA.2.86 and descendant JN.1 in Denmark: a nationwide observational study.Lancet Infect Dis. 2024 Sep;24(9):964-973. doi: 10.1016/S1473-3099(24)00220-2. Epub 2024 May 15. Lancet Infect Dis. 2024. PMID: 38761806
-
Distinct patterns of SARS-CoV-2 BA.2.87.1 and JN.1 variants in immune evasion, antigenicity, and cell-cell fusion.mBio. 2024 May 8;15(5):e0075124. doi: 10.1128/mbio.00751-24. Epub 2024 Apr 9. mBio. 2024. PMID: 38591890 Free PMC article.
-
Persistent T cell-mediated immune responses against Omicron variants after the third COVID-19 mRNA vaccine dose.Front Immunol. 2023 Jan 23;14:1099246. doi: 10.3389/fimmu.2023.1099246. eCollection 2023. Front Immunol. 2023. PMID: 36756112 Free PMC article.
-
COVID-19 Vaccination Recommendations for 2024-2025 in Korea.Infect Chemother. 2024 Dec;56(4):453-460. doi: 10.3947/ic.2024.0142. Infect Chemother. 2024. PMID: 39762924 Free PMC article. Review.
Cited by
-
Role of senescent CD4+ T cells in breakthrough infection of the new variant strain of SARS-CoV-2 in elderly patients.J Transl Med. 2025 Jul 3;23(1):737. doi: 10.1186/s12967-025-06756-0. J Transl Med. 2025. PMID: 40611132 Free PMC article.
References
-
- Chenchula S., Chandra M.B., Adusumilli M.B., Ghanta S.N., Bommasani A., Kuttiappan A., Padmavathi R., Amerneni K.C., Chikatipalli R., Ghanta M.K., et al. Immunogenicity, clinical efficacy and safety of additional second COVID-19 booster vaccines against Omicron and its subvariants: A systematic review. Rev. Med. Virol. 2024;34:e2515. doi: 10.1002/rmv.2515. - DOI - PubMed
-
- Moustsen-Helms I.R., Bager P., Larsen T.G., Møller F.T., Vestergaard L.S., Rasmussen M., Hansen C.H., Christiansen L.E., Gubbels S., Trebbien R., et al. Relative vaccine protection, disease severity, and symptoms associated with the SARS-CoV-2 omicron subvariant BA.2.86 and descendant JN.1 in Denmark: A nationwide observational study. Lancet Infect. Dis. 2024;24:964–973. doi: 10.1016/S1473-3099(24)00220-2. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

